
-----
303 Stainless Steel Passivation Problems
[editor appended this entry to this thread which already addresses it in lieu of spawning a duplicative thread]
Q. We had some 303SST parts come in that were noticeably darker then the other lots received. We also noticed on several what appeared to be fingerprints or blotches of discoloration also.
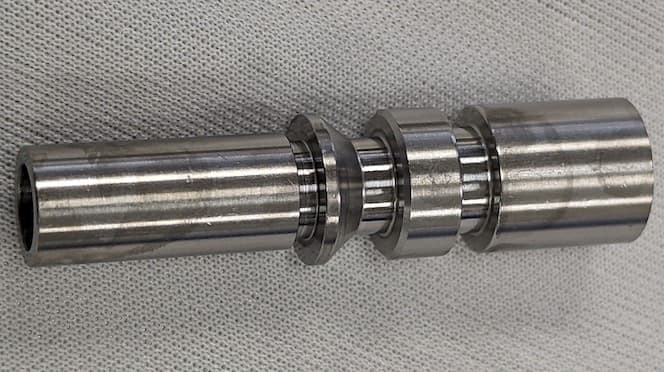

Could this be a result of the parts not being properly cleaned or from a material composition difference?
Mac McConnell- Redwood City California
April 10, 2023
A. For starters, there's the usual issues with free machining (high sulfur) grades like 303, they need the proper passivation process, either nitric type II aka Nitric 1, or an alkaline pretreatment prior to citric passivation. Extra surface sulfur could explain the general darker color.
The blotches are probably exactly that, fingerprints and lack of sufficient precleaning. The alkaline cleaner ought to be able to take care of those, or switch to handling the parts with gloves only.

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

May 1, 2023
A. Pitted, dark, stainless steel passivation: I passivated some aviation bushings Half came out pitted and blackened the other half pretty shiny finish. I informed the customer and they were upset that I ruined their parts. I never understood what happened but I was confident the the chemicals did not lie, they had two different alloys.
Former Metal Finisher, I crossed over to the "DARK SIDE" now I work for the state's environment department.
- New Mexico
April 14, 2024
Tip: This forum was created to build camaraderie through sharing of tips, opinions, pics & personalities.
The operator & readers who are here for that often won't engage with anonymous posters.
⇩ Related postings, oldest first ⇩
Q. I have some 303 stainless steel 3/8" pins that will see salt water and fresh water environments. These parts were passivated. I subjected them to 500 hours of salt fog testing and they rusted mostly at the ends where they were machined/cut. I also ran 304 pins at the same time and they did not rust. I was told that 303 should not rust if they are properly passivated. How can I tell if they are properly passivated in the future? Is there a test I can run?
Sid Tryzbiak- Tulsa, Oklahoma
2003
A. Most of the 303 that I saw at two shops was "free cutting" and was normally 303Se. This proved to be much harder to passivate than plain 304. Salt spray of a larger sample would be my approach to telling if it were properly passivated. copper sulphate ⇦ on eBay or Amazon [affil link] testing can be done fairly easily "in house" also. Ferroxyl is a far more discriminating test, but it is more sensitive than most peoples requirements. You might look into doing a citric acid passivate in house with a proprietary product such as Stellar Solutions [a finishing.com supporting advertiser]. For the ultimate, in my opinion, have them electropolished.
James Watts- Navarre, Florida
A. Sid, dump the 303. Use 304 or 316, or some other stainless that isn't free machining. The small manganese sulfide inclusions that give 303 its easy machinability mess with the corrosion resistance.
If you're stuck with 303, try passivating it with a Type VIII solution, rather than the type II your vendor is likely using. We've had better luck with VIII.

Lee Gearhart
metallurgist - E. Aurora, New York
Multiple threads merged: please forgive chronology errors and repetition 🙂
Challenges with Passivation of 303S
Q. I have found this forum to be extremely useful over the last several years. I have read numerous posts and the FAQ in this forum related to passivation problems - especially those related to "unexplained" frosting, burning, etching, and discoloration. Problem: 303(S) displays "frosted" (light etch) after nitric/dichromate (QQ-P-35C (canceled) [link is to free spec at Defense Logistics Agency, dla.mil]) passivation. Extent of frost varies (apparently at random) from piece to piece and load to load. Frosted appearance varies from ~100% to 0% surface coverage. Several loads of other 303(S) parts were successfully processed on the same line on the same date with identical processing (automated line) with no problems.
Some of the parts were process in a smaller "hand" line at another site that display the same problem. Results suggest the same mechanism is at work. However, the parts have less "frost". (Possibly related to the random nature of the problem, possibly to some unknown difference in conditions?)
One of the suggestions on this site referred to soaking the parts in alkaline solution prior to processing. We have tried one hour soaks in both alkaline cleaner and 5% NaOH solutions, both heated to 140 °F, with no visible change in results.
We are a captive shop. We are using many of the "free machining" CRES alloys. At this time, it is not an option to change raw materials. We are ordering several forms of 303 raw stock (hot rolled, drawn, cold rolled, etc.) to see if we can identify a specific form of the material that seems to give us problems.
We recently had a similar, though much more catastrophic episode with some 416 parts. The attack was far more aggressive, but reminiscent of this problem in that the attack appeared to be random, varied from piece to piece, and from load to load. The variance in the attack was more severe, varying from nearly complete destruction of the part to no visible signs of attack.
Questions:
1. Precisely what is the mechanism of the attack on the sulfides? Is it removal of the sulfides from the surface or a secondary reaction forming (sulfuric or sulfurous?) acid which then attacks the material?
2. What are the accepted methods for prevention?
3. My company works on military hardware and is currently working to AMSQQP35 [canceled]. citric acid is sometimes quoted as a potential fix but is not approved (according to the way I read the spec). If other shops are using citric acid , are they doing so on military hardware? If so, how do you conform to the spec and still use citric acid ?
Jeff WaltonProcess Engineer - Sherman, Texas
2003
A. Jeff, don't use Type II. Use Type VIII. It's cheaper, it's less hassle, and it works for both 303 and 416. We switched several years ago and haven't had any more etching.
You may have to run some tests so as to have documentation to satisfy some of your customers. Type VIII isn't listed as a recommended bath for 303 or 416 in the back chart of QQ-P-35. Yet if folks get porky about specsmanship issues, you can likely point to the first cancellation notice of QQ-P-35, which sent you to ASTM A967. That spec allows various combinations of bath, time, and temp provided you pass the testing. In ASTM nomenclature, I recommend Nitric 4 rather than Nitric 1.

Lee Gearhart
metallurgist - E. Aurora, New York
A. I agree with Lee's comments about using nitric Type VIII if you have to use nitric acid for these grades. A number of our customers have had success with this.
The most accepted theory about what is happening is that the sulfides bloom to the surface through the grain boundaries. This causes a number of problems and reactions with the nitric acid solution, especially after you have done a number of parts in the solution and pulled some sulfide into the solution.
If you read the manuals on this most everyone agrees that you need to pretreat with alkaline before passivation of these grades. This is not only to prevent the frosting, but also to get better passivation and prevent post passivation discoloration from the sulfides. Talk to the steel companies.
Military has approved the use of ASTM A967 for passivation of their parts, since the cancellation of AMSQQP35 [canceled]. If it is aerospace, however, you must use either AMSQQP35 [canceled] or AMS2700, which is replacing AMS QQP35. AMS2700 allows the use of nitric or citric acid formulations.

Lee Kremer
Stellar Solutions, Inc.
McHenry, Illinois

A. Lee is right. Citric will cure your ills. I like the caustic hotter, 160 °F min. If someone has their undies in a bundle over the spec we'll passivate with citric first and finish with the Type II which we must cert to. Silly, but meets customer specs and gives them what they want, passivated parts.
Jon Quirt- Minneapolis, Minnesota
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. We are a small CNC machine shop, running mainly 316ss,17-4PH, and XM-27. As a service to one of our larger customers, we run a small amount of 303ss. Because of the fact that most of our parts are passivated in-house to QQ-P-35C (canceled) [link is to free spec at Defense Logistics Agency, dla.mil] either type II or Type VI, we are a little apprehensive of passivating 303 after reading some of your articles on the problems that may be encountered, with thta alloy.
What we are wondering is there any test we can run on parts made from 303ss to check for sulfur, which we believe may be the variable that interferes with passivation?
We do run a sample part through prior to running the entire batch, and as a precaution, all parts to be passivated are ultrasonically vapor degreased prior to passivation attempting to remove any sulfur contaning oils, but we are concerned about sulfur in the metal itself.
Robert Prichardheat treat / passivation mgr - Chadwicks, New York, USA
2004
A. Good for you you are being pro-active rather than reactive. Yes be concerned with 303. More so if is nicely machined as this will smear the sulfur over the surface. You can try the AAA process, Alkaline/Acid/Alkaline, NaOH 5% by wt. 160 Min 60 minutes/Type II/ Again alkaline. Or make life simple and use Citric passivate from Lee at Stellar Solutions [a finishing.com supporting advertiser]. If you must spec to the obsolete QQ-P-35 then pre-passivate with a good long citric. It will complex the sulfur. (Lee will most likely answer also, Hello Lee)
Jon Quirt- Minneapolis, Minnesota
A. Jon is correct. If you pretreat the 303 with hot alkaline solution you should have no problems. We have many customers doing this with complete success. There is a considerable variation lot to lot with 303 and sometimes you can get by with minimal treatment, but you need to treat like Jon says or pretest every lot. (Hi, Jon!)

Lee Kremer
Stellar Solutions, Inc.
McHenry, Illinois

Passivate 303 free machining stainless per ASTM A380
Q. I have a part that calls for passivate IAW ASTM A380
, Code A.
The material is free machining 303 stainless per ASTM A582
.
Code A (Table A1.1) of ASTM A380
seems to be for pickling (descaling, i.e.heat treat scale or whatever). Our part is not heat treated, it is 100% machined surfaces, nice and clean.
Question: Code A doesn't seem like the best choice for 'passivate'. Would a more appropriate Code per ASTM A380
be J, K or L (Table A2.1)?
Quality Engineer - Portland, Oregon, USA
February 9, 2015
A. Kelly,
Passivation in A380 refers to the processes in Table A2.1 part II, which is codes F-M. Code A is in Table A1.1.
You are correct, Code J/K/L is stated for "200, 300, and 400 Series free-machining alloys", which 303 falls into. Although in my opinion, codes K and L do not make sense for what they are supposed to be doing and should not be used for anything.
The 2013 revision also opens the door to using passivation per A967 to meet the requirements, which in my opinion is much better than anything from Table A2.1, especially if you choose to use citric acid passivation with alkaline precleaning rather than nitric acid with added sodium dichromate. Your process would be much less hazardous in that case.
adv.
Let me know if I can help.

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

Q. I have a client who faces a problem while passivating/electroplating high sulfur grades (free machining grades). My hypothesis is that this sulfur leaches out rapidly and reacts with the solutions forming precipitates / complexed products?
Perhaps someone has more insight on the precise physical/chemical mechanism?
Metallurgical Engineering - Murten, Switzerland
December 13, 2018
A. Hi Jean-François,
A couple of pictures would help here, to see exactly what you are talking about.
I have had problems with high sulfur compounds in the past. The hypothesis here is that the sulfur stringers are attacked in the alloy preferentially, which then sets off the chemical reaction that causes etching.
To get over the problem we treated the alloys as if they were high carbon alloys, such as 440C, using a good, strong alkaline cleaning process and using fresh passivation solution. This solved the problem, without us truly getting to the root cause, which is what often happens in a production environment.
Aerospace - Yeovil, Somerset, UK
|
|
Q. Hi Brian, Metallurgical Engineering - Murten, Switzerland A. Sulfur left exposed on the part will react to form sulfuric acid in a nitric bath; nitric acid is an oxidizer and you end up with NO2 and sulfuric acid, which is damaging to stainless and having that reaction take place right there on the surface of parts is just begging for scorch.  Rachel Mackintosh lab rat - Greenfield, Vermont
Regards,  Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted can be retained for immediate answers or long term project help December 2018 |
|
|
Metallurgical Engineering - Murten, Switzerland December 14, 2018 A. Thank goodness for ASTM A967 section 7.1.1.4 !  Rachel Mackintosh lab rat - Greenfield, Vermont |
A. A 1% each citric acid and sodium nitrate solution is not very likely to actually passivate stainless steel. 4 wt% minimum citric acid is the industry-recognized mixture. A380 code N falls under the heading of "cleaning" rather than "cleaning and passivation".
As far as free machining grades go, yes, the sulfur is a source of various problems. In the old nitric acid passivation days, it was "solved" with the addition of dichromate to the bath. These days we recognize that a good alkaline bath pretreat usually gets enough sulfur out of the surface that it won't be an issue.

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

![]() Hi Ray,
Hi Ray,
I 100% agree with you when it comes to standard operation of Citric tanks, and a good deal of what I know about their unique benefits comes from your posts over the years.
This all started with one particular large batch of parts from a single heat lot of 416 that one day (cut on first shift) was running fine, and the next (cut on second shift) was burning black in the tank we historically use for 416, from a recipe given verbally to my mentor years ago by a technical rep at Carpenter Steel. And it usually works!
We then tried the parts in our standard Code N hot citric 4-6% tank, which we use for a range of alloys, and they literally disintegrated. Like, there was NOTHING left in the baskets but bubbles.
By this point, I had two senior managers and a VP hollering on the phone for parts as I'm trying to squeeze in questions about the run conditions, cutting fluid, deburring op, etc...
That's where we ended up throwing a Hail Mary pass- um, I mean- trying the weak cleaning solution. All our work is tested post-process, and the parts passed 24 hour water immersion, so we continue to use it.
I'd definitely reiterate the importance of the statement 'to pass specified test requirements', and definitely NOT to assume that the method worked without running water immersion on it!
It's just one more weapon in the arsenal against PITA parts :)

Rachel Mackintosh
lab rat - Greenfield, Vermont
Ed. note Feb. 2015: Readers may also be interested in thread no. 639, "Passivation of 303 stainless steel".
Q, A, or Comment on THIS thread -or- Start a NEW Thread
