
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Need 20µ gold or silver coating on 50µ dia. spherical magnets
Q. I am a physicist at the University of Southampton. We have some NdFeB (more specifically Nd-Pr-Fe-Co-Ti-Z-B, and mostly Fe) based ~60micron diameter spherical magnets (MQP-S-11-9-20001, supplied as a non-magnetised powder) that we want to coat in 20-30 micron thick gold in order to do a scientific experiment. (silver would also be an option, maybe also copper). This relatively large amount of thickness is very important. For the material we want the highest conductivity possible without the surface likely to get covered in a less conductive oxide/sulphide layer.
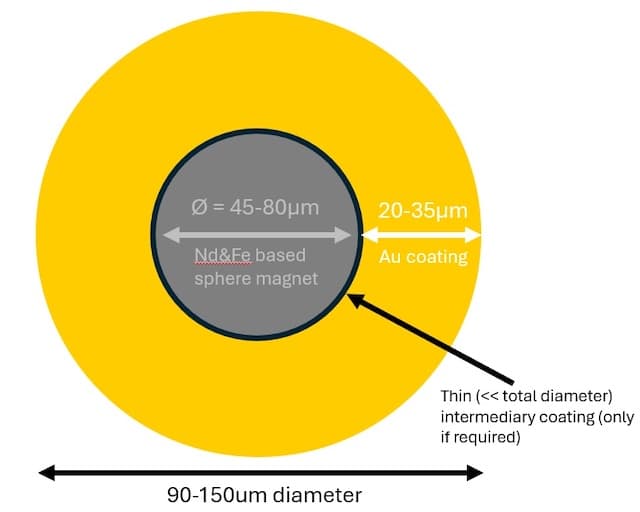

What is the best way of doing this? I have approached some magnet-making and plating companies but they have all said the magnets are too small for them to coat. I am trying to work with some colleagues in the Chemistry department, but they only have experience synthesising silver nanoparticles, a little plating expertise, so this is a bit too big a scale for them. We have only managed so far to create a thin (<2 micron) layer of silver on the magnets, and the thicker layers are incredibly bumpy, no longer a smooth sphere at all.
As the magnets are very small (and we would ideally like to coat several at once so we have spares/a range of sizes), from my reading it seems like an electroless method would be best, and perhaps an autocatalytic growth method is the only way to get the thickness + smoothness required? But maybe there is a way to do some kind of barrel method with a very fine mesh? I am very much not an expert or any kind of chemist so all advice/recommendations welcome.
Scientist - Southampton, UK
February 14, 2025
privately respond to this RFQ
Ed. note: As always, gentle readers: technical replies in public and commercial replies in private please (huh? why?)
A. Hi, Dr. Marion.
Silver and copper will acquire non-conductive tarnish, so gold is best. That thickness is probably too think for autocatalytic gold (although the thickness would probably keep building, it will probably not be remotely smooth at that thickness). I think you'd be limited to doing electrolytic gold plating.
There are commercially available "vibratory plating barrels", and shops which offer plating services utilizing them. They are not actually rotating barrels, but a sort of vibrating pan. You could try googling that.
However, you may find that your powder is too fine for them. You may not find a mesh large enough for water to flow through, but small enough for 45 micron balls not to fall through or get stuck in. In that case I think the way it could be done is with a layer of the powder sitting on a metal screen connected to the cathode, in turn sitting on a vibrating plastic pan.
If you cannot find anyone to do it, it may require research efforts. I have only seen powder electroplated in one facility and it was a challenge. Due to the very large surface area, chemical reactions occurred almost explosively fast. If you can't find anyone to do it, try reaching out for Dr. David Lashmore at Nanocomp Corp. in New Hampshire, USA. He was in charge of the only plating on powder project that I've seen.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
![]() Thank you Ted, I will look into vibratory plating services and also get in touch with Dr. Lashmore.
Thank you Ted, I will look into vibratory plating services and also get in touch with Dr. Lashmore.
Q. How vigorous is the vibration? In our coating attempt so far we strangely lost most of our larger magnets and were only left with ~10 micron size ones, possibly because they were broken up by the solution being agitated.
We can coat mostly in silver with a thin layer of gold on the top if that is any easier to achieve the total conductive thickness + lack of tarnishing?
- Southampton, UK
February 17, 2025
A. Hi again. I'm confident that the vibration can be whatever you want it to be, but the idea is to jostle the powder around so the top gets plated then what was on the bottom, then what was on the sides, and repeat.
I don't think silver is superior to gold for build or vice versa; but when we want thick plating its often best that it not be just one type because the crystal structures keeps growing and the deposit is less satisfactory than half thicknesses of two different metals.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread