
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Sterling silver is rapidly tarnishing
April 17, 2019
Q. I am doing silver plating. Composition NaCN 30g per liter & 0.5g SPC Temperature 70 degree. Finish is good after rinse 1. sulfuric rinse rinse2 anti-tarnish chemical dip again rinse3 rinse4 rinse5 then EP lacquering. Sometimes I face yellow shade -- why do I face this and how to prevent this? Please help me.

vasanth kumar
Gold plating art studio LLP - Chennai,Tamil Nadu, India
A. Hello,
You may want to improve the rinsing post silver plating,
also keep the antitarnish bath in pristine condition.

Khozem Vahaanwala
Saify Ind
Bengaluru, India

June 15, 2019
A. Hello,
In addition to what Khozem replied, you want to make sure your carbonate level in the silver bath is not too high as that can cause yellowing of the silver deposit. After silver plate, rinses and dips, dry and store in a clean environment. If the deposit doesn't yellow in a day or two, you can eliminate the silver bath as being the culprit. I have also seen dull and yellowish finishes from a bath that needs a carbon treatment.
Electronics plating - Winston-Salem, North Carolina USA
June 28, 2019
A. Hi,
When you use NaCN the silver will be yellow. If you use a KCN will give you right tarnish.
Regards

Anders Sundman
4th Generation Surface Engineering
Consultant - Arvika,
Sweden
July 19, 2019
⇩ Related postings, oldest first ⇩
Q. Respected sir,
I am facing one problem in silver plating Ours is a conventional silver plating bath contains 150g/l KCN & 25 g/l Ag. We have improved our acid conc. of acid cleaning to 25%. Alkaline cleaning bath conc. is also increased to 8%. Still we are getting yellow patches. Our process sequence is as follows: hot soak (5 min), anodic (3 min), rinsing, acid cleaning, rinse, copper plating (Rochelle), cyanide dip, silver strike, silver plating, rinse, passivation (chrome), rinse, hot rinse.
Please tell me what could be the reasons. Also please write us ideal conc. of plating bath & cleaning conc. to get quality finish.
Thanks regards.
Avinash P BarveIndia
1999
A. Hi,
First you have to check your brighteners; if you don't use, you must.
You can give the piece a nickel coating after the copper.
The ideal bath of silver is 125 to 150 gr/L. KCN 26 to 32 gr/L. Ag
Please if you have any result, post it.
Jamyl.
Jamyl D'AngeloLima Peru
A. You might want to try turning off the heat on your final rinse. On matte finish cyanide silver this is a must to eliminate yellowing. It takes longer to dry the parts, but the appearance is much better. Also, are your spots appearing after you handle them? This can also be a trouble spot.
Phil Pace- Tulsa, Oklahoma
A. DEAR SIR, BE ADVISED PLEASE TO USE BEST QUALITY OF brighteners AND MAINTAIN PROPER CONCENTRATION OF brighteners. UTILISATION SYSTEM OF CYANIDE WILL SOLVE YOUR PROBLEM.
SHAH JANATAHMEDABAD/ GUJ/ INDIA
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. I require a good anti-tarnish finish for silver. We are etching the surface off sterling silver which leaves a brilliant matte white finish on the metal. To date the finishes we have used have a yellowish discolouration. Has anyone got an idea of how to maintain a good finish with no discolouration.
Chris Stocktonphoto etching - England
1999
A. You might want to contact Technic. They have several anti-tarnish products that may help you. I believe they have an office in the U.K.

Jim Conner
Anoplex Software
Mabank, Texas USA

1999
----
Ed. update April 2019: We appreciate Jim's reply from back in 1999, but times have changed, and time has demonstrated that we should no longer recommend brands or sources on these pages (why?)
A. Hi Chris.
If you use NaCN so can the surface tend to a yellow finish and get a very bright finish for a while but after a few weeks display a yellow finish. Which also happens when the carbonates is too high when you use KaCN, or the brightener is too high in the silver solution.
Regard Anders S

Anders Sundman
4th Generation Surface Engineering
Consultant - Arvika,
Sweden
1999
2000
A. Dear Chris Stockton
Options are available. Before I give options, the following are prerequisites:
1. The component should be cleaned thoroughly with deionised / demineralised water (preferably warm water, about 60 °C). Prior to this wash you can have running tape water wash. This cleaning in DI/DM water would not leave any residue of water on the plated area (if you use ordinary water, the dissolved salts present in the water will be left on the plated area once the water evaporates).
2. Keep the component protected from acid or sulfide environment. These would lead to the tarnishing of silver. By this way, the tarnishing period can be extended, may be matter of hours or maximum days.
Now the Options:
Basically three options are available. Choice would depend on the type of application the plated job would go for (industrial / ornamental / decorative etc):
a) Organic protection:
The component will be dipped into a solution containing some organic molecules. On dipping, there will be monomolecular layer formation which will protect the component from tarnishing. Limitation: Temperature. Higher the temperature of the job functioning, shorter the life of protection. The organic molecules will disintegrate.
b) Inorganic protection:
b.1) This will be electrolytic plating process of depositing a fraction of chromium over the silver. There will be a reduction in the colour of the finish, but it will be insignificant. But a very good protection (would depend on the thickness of Cr). Limitation: Chromium disposal. There might be some reduction in surface conductivity too.
b.2) This process has also been proved. Electrolytic plating of Rhodium. This is also found to be a very effective anti-tarnish coating. Limitation: High cost.
c) The other one could be, not very sure, you can try clear lacquers, if your application permits. Acrylics or PU. Now these are available in electrophoretic formulations, so that one can control the thickness like plating.
Try them. Good luck.
With Best Regards,
Dr.K. Murugan- Mumbai, India
A. I have been looking for an anti-tarnishing agent for our pure silver leads that resists temperature, doesn't yellow and allows soldering without toxic fumes. I found Everbrite a chemical coating from Everbrite [a finishing.com supporting advertiser]. They are in Reno, Nevada.
This might work out well for you.
Sincerely, John
John J. Campanella- Bohemia, New York
2000
RFQ: We are a major Electroplater in Bangalore (INDIA). We are in need of chemical for anti tarnish on silver plating.
Therefore please send details of the said chemicals at the earliest along with minimum order quantity and rates.
M.K.MAHESH [surname deleted for privacy by Editor]Bangalore (INDIA)
2001
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
Q. We have continuous LED silver plating.The step are AD->ED->Acid(HCl)->nickel-plate->Cu-Plate(thickness about 3-4 micron)->Ag strike (thickness about 0.2-0.3 micron)->Ag plate (thickness target about 3-5 micron)
The speed is 1.7 meter per min.
Now we have problem about silver plating surface that have many yellow dot .It look like rusty color.
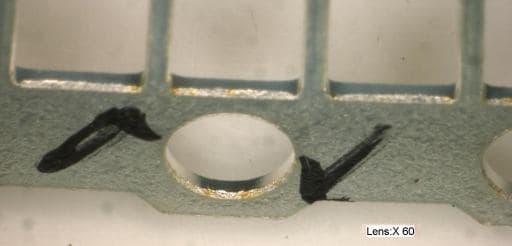
I am not sure this problem concern material. Please give us any suggestions.
Chookiet Jaroenchung- Ayutthaya, Thailand
2005
? Maybe, I read your procedure improperly but The Nickel plate should be diffusion barrier between the silver plate and copper plate?
Hamilton Solidum- Mays Landing, New Jersey, USA
2005
? Hi,
You must explain more about you pretreatment and also you chemistry in the silver solution, Can it be that you use sodium cyanide in the silver solution?
Regards

Anders Sundman
4th Generation Surface Engineering
Consultant - Arvika,
Sweden
2005
Sterling silver is rapidly tarnishing
Q. Hi, I have a small jewelry store where I make sterling silver jewelry. I've been in this location for 3 years and tarnishing has always been a problem. Unfortunately, over the last few months it has increased substantially. My new sterling jewelry needs to be cleaned with a tarnish remover paste every 3-5 days. All the sterling looks gold and then turns ugly brown.
There is a Subway restaurant, a dentist, a video store and a paint store in my building. I cannot smell paint ever, can smell baking bread all day long, everyday.
I have installed an ionizer, have a window ac running with the door closed. Help.
jewelry designer - Franklin, MA, USA
August 14, 2009
|
A. Overall, the best solution would be to move to a better location. - Navarre, Florida August 18, 2009 A. Try 3M silver protector strips (USA product). Hope it helps and good luck! Goran Budija- Zagreb,Croatia August 19, 2009 A. Lisa, Fellow Plater - Syracuse, NY USA August 20, 2009 |
A. I'm not certain how you do business but a friend of mine makes a living making custom hardware for muzzle loaders out of brass and silver.
He was having the same problem as you and it was costing him gobs of time and money. In the end he made up a set of samples for his display and had them rhodium plated. Then when some one wanted a part he would show them the sample and then make the part to demand. It worked great but a lot of his customers would come in from out of state and would want the part RIGHT NOW! He found that he was losing sales.
I'm not sure how he came up with this because he's not the sharpest tack in the box but he bought a huge fish tank six feet long two feet deep and on foot wide with one foot square pieces of glass to completely cover the top. He put his items for sale in the tank and filled the tank with argon gas. If people want to touch he gives them the rhodium plated parts from under the counter. If they want to see the true color they can gaze at the fish tank. If they give him money he will remove one of the one foot squares and fish out a part. Before he fully closes the top he bleeds in a foot or two of argon and closes the box up.
There are a couple simple rules he follows. Before a part goes into the box he only handles it while wearing cotton gloves and he cleans the part with a jewelry polishing cloth before they go in. He claims he has had some parts in the tank for over a year with no tarnish. Claims he spends about a hundred bux a year on argon. Go figure? :o)

Rod Henrickson
gunsmith - Edmonton, Alberta, Canada
August 22, 2009
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. We are manufacturers and retailers of silver products. We lacquer our products, however they are tarnishing very fast. Can anyone tell us what would be the best possible technique and chemicals to avoid this. We are Delhi, India based
Tulu Patnaiksilver workshop employee - Delhi, India
February 24, 2011
RFQ: We need to use anti-tarnish agent on the silver plated parts, which will be suffer the SMT reflow with the highest temperature up to 260 °C. Is there any anti-tarnish agent can work for this?
Weijie Guo- Xiamen,China
August 20, 2012
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
Q, A, or Comment on THIS thread -or- Start a NEW Thread
