
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Growing Plating Crystals (Nickel trees) as Jewelry and Art
Q. I discovered this on the web the other day and am very curious about how deposits like this could be grown. Supposedly these are cast-offs from a nickel plating operation, they form on racks that hold objects to be plated. I would like to know if anyone is familiar with deposits like these, knows the process which creates these, and could speculate what makes the deposits grow in this manner. Thanks all.

enthusiast - Doylestown, Pennsylvania, USA
2007
A. They are "nickel trees" and many platers have them decorating their desks, John. Indeed they are no work at all, but a headache. Anyplace where metal is exposed on a plating rack the nickel will start to plate out and just keep plating out. You may be able to bend a piece of wire before plating and get a particular artistic shape.
They are probably bright because of the brighteners in the nickel plating solutions. There may be a more scientific explanation for their shape, but I think it's just the geometry of the current flow: As a nodule begins to grow, the outside of that nodule is closest to the cathode and continues to preferentially grow. I'm not sure why it stops, but I'd guess that when the power is turned off overnight the nodule tarnishes and passivates so that it is not as conductive, so seeding starts at new points and those nodules grow. You didn't say where you found this photo, but it look like we can credit theodoregray.com.
There is an article on the subject, under the title "Nickel Growing in Trees", in the April 2006 issue of Popular Science. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Thank you Ted for the generous response. You mentioned that the geometry of current flow might have an effect on the growth of the nodules. Anywhere I can learn more about this-- the geometry of current flow in the plating process?
Am I correct in assuming similar formations can occur with other metals? Would a different metal create a different nodule structure? I don't know the scale of the sample but roughly how long do these take to develop? Thank you for cutting me slack on the copyright, your credit to theodoregray.com is right. -John
enthusiast - Doylestown, Pennsylvania, USA
2007
A. The amount of plating deposited is proportional to the current flowing to that point, and the current will take the path of least resistance. I think nickel makes the most aesthetic trees, but I've seen huge blobs of copper. When growing by accident through the splits in rack coatings, the trees probably take a few weeks to build to what you see. As for scale, the larger nodules are about the size of a pea. I edited my reply (before I received your followup) to make reference to a Popular Science article.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. You can grow shapes a lot bigger than a pea by using stainless steel wire that you have sharpened the points on as the cathode. You could wrap 2 - 3 in one group facing away from each other.
It results from the extremely high current density at the point plating many times faster than a part. Many times it is caused by a sharp bur or conductive spec on a part. It is common at the intersection of a part and its masked edge.
- Navarre, Florida
Sorry that my reply was confusing, James. Yes, these trees can be good paperweights and are much larger than a pea. John said he had no sense of scale from the photo and I meant that the size of the largest individual rounded bumps or nodules in his picture might be about the size of a pea.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. I once did a fun little project.
I had some silver wires from some old spent pH electrodes somebody was throwing away...with the idea that I'd get some solid silver billet electrodes, I put them in a 500 ml Hull Cell, filled it with cyanide silver plating solution, and turned the power supply on just as low as it would go. Then I went out of town for 6 days.
When I came back, I had grown these ... silver twigs. They had a grainy, "organic" appearance. Really sharp looking. I made each one into a pendant (I fiddle around with jewelry sometimes) that got lots of admiring comments.
You could do the same thing with an acid copper solution (far less toxic) and get all kinds of fun shapes by plating little twists of stripped bell wire in the same way.

Dave Wichern
Consultant - The Bronx, New York
A. Take a bunch of copper wire and twist them together for a couple of inches.
Open out the strands and trim to a rough cone.
Plate with nickel for a day or so(chrome works very well too).
Welcome to the world of platers xmas trees. But don't let the boss see you, he will want one.
Or; Take your nodules (copper is good for this) Choose one with a relatively flat back. Solder on a small loop of wire and gold plate. Great pendant for your favourite lady.

Geoff Smith
Hampshire, England
Q. Do the deposits have to happen at a burr or edge? Let say I was plating a smooth hemisphere. Would it eventually produce this kind of growth? If not, what if the surface of the hemisphere was perturbed in some way, how would I perturb the surface to begin producing deposits?
John Bersethenthusiast - Doylestown, Pennsylvania, USA
2007
A. Most plating will eventually grow very course and nodular, especially if the solution has no brighteners, and especially if the current is too high for smooth plating. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Hi,
I've posted on the forum before [see also my letter 45277, "Plating Plastic Honey Bee Comb"] on the subject of nickel trees or aggregate deposits created by extended bouts of plating. I think I now better understand what factors influence where deposits form (position of the anode, the degree of curvature of the cathode object.) I'm attaching an image of the kind of deposition that I write about.

Now I'd like to try to learn a bit more about what determines the actual shape of these deposits. I'm hoping to see if some variation in the electrolyte would make these deposits more branchy or dendritic. I believe the shape of these deposits (the degree to which they are either nodular or branchy) has to do with the throwing power of the electrolyte. Is this correct, does the throw of an electrolyte have an effect on the shape an aggregate deposit takes? Are there baths for copper or nickel plating that have limited throwing power?
John Bersethenthusiast - Doylestown, Pennsylvania, USA
2007
A. The second lot of pictures are typical of what we call a dog-bone effect. Since current always takes the path of least resistance, the nodules growing on the edges is typical of nickel.

Khozem Vahaanwala
Saify Ind
Bengaluru, Karnataka, India

A. John, these things look like tumors! What are you going to do with them?

Sheldon Taylor
supply chain electronics
Wake Forest, North Carolina
A. To research and predict the growth of nodules, I suggest that you start with the mathematics of chaos theory and finite element analysis.
Let us know how you get on.
Good luck.

Geoff Smith
Hampshire, England
Q. Hello,
I was wondering if John Berseth had any further results or some research to share?
Best,
Gregory
- Geneva, Switzerland
October 17, 2010
A. Hi, Gregory. John hasn't been back for a few years, and most people's email addresses go out of date quicker that that. People usually come back when they do an internet search and see their own name. Meanwhile, please describe your situation or post your own question, and chances are good that you'll start an interesting dialog, while also keeping the page fresh for good search engine placement so John will eventually stumble upon his posting again :-)
Letters 37080, "Trees on stainless steel acid copper plating process" and 25979, "Recovering silver via electroplating" also address the subject of "trees". Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
October 18, 2010
October 24, 2010
![]() Hi Ted,
Hi Ted,
Thank you for the reply.
Here is my project:
I have this architecture model made out of bronze casting. (For information, its the Palace of Culture and Science in Warsaw).

And next Tuesday I will bring it to a local electroforming company here in Poland. There it will be copper plated for as long as possible to create dendrite formations similar to the photo of John above in this discussion.
Now based on the model, what are the advices you could give me? Would it be interesting to have the model rotating while in the bath? or just change the position once in a while during the process? Are we talking about 3 days of plating? 3 weeks? What are the crucial things I should tell the plater?
I know I will get answers by trying, but I was wondering what was your opinion about it.
Take care,
Gregory
- Geneva, Switzerland
by Jay H. Newman

on AbeBooks
or eBay or
Amazon
(affil links)
A. Hi, Gregory.
Realistically, the hands-on copper electroformer is probably going to know more about this than I do, and probably won't appreciate 3rd-party advice from someone who has never seen his shop, his tooling, or his capabilities anyway :-)
But, yes, I think it should be periodically rotated every couple of hours. Judging more from when good electroforming goes bad than from deliberate attempts to build copper trees, I think 2-3 days of plating in a bath with no brightener will produce the desired results. Other readers may have a better feel for it. Best of luck!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
October 25, 2010
Q. Hello Everyone, this is exactly what I have been looking for.
My one simple question is, could you do this kind of electroplating using a battery pack of AA or 9V batteries?
I am working on robotic growth, and intend to use electroplating to allow the robot to grow.
Please let me know if anyone knows how to electroplate using batteries.
Thank you!
- Winnipeg, Canada
January 12, 2011
A. Hi, Jake.
Plating with batteries is not much different than plating with a power supply except for the inability to control the voltage (without inserting power-wasting rheostats or resistors in the circuit) and the fact that a battery won't last long.
Depending on what you are plating, and with what, something like 3 volts will be enough. But recognize that a battery and a plating tank are pretty much the same thing operating at reversed polarity. Your battery must provide enough voltage and then some to force the plating tank to run as a battery in "the wrong direction", and you'll appreciate that a battery can't power any more plating than empowered by its own deplating.
For a quantitative answer to the idea I'm presenting, see Faraday's Law of electrolysis, from which you can calculate how many ampere hours are required to deposit a given amount of plating. Penlight and 9V batteries only have the power to do a very little bit of electroplating. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
January 12, 2011
Q. Thank you so much for your reply and the great information about the Faraday Laws of Electrolysis.
Could you take a look at my concept for robotic growth here and let me know if you think it would work?
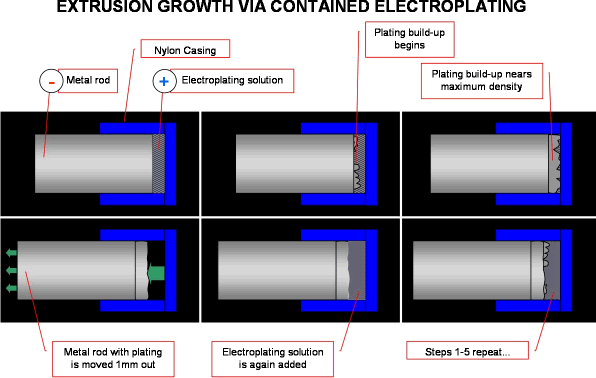
I am trying to create a rod by "growing" it using electroplating, each time the buildup is significantly thick, the rod is moved out so as to expose more area for the rod to plate up - in theory, would this work?
Jake Middleton- Winnipeg, Canada
January 13, 2011
A. Hi, Jake.
Well, yes, it could work "in theory". But in practice? Sorry, but I doubt it. Most plating has a very limited thickness due to such factors as: the tensile or compressive stresses become so severe that the object lacks real mechanical integrity; the plating becomes too porous as small lumps grow on big lumps and you don't get plating down between them. With a carefully engineered cell, with plating solution jetted at the surface, it might be possible to repeat your steps a few times and build a few mm of rod, but as a practical way to do continuous extrusion for anything but very small robots, I don't think it looks very hopeful. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
January 13, 2011
How to cultivate large electroplating nodules, for art

Q. I've never done any electroplating, but I'm struck by the beauty of some of the larger metal nodules you occasionally see photos of. For example:
www.theodoregray.com/periodictable/Samples/028/s14s.JPG
commons.wikimedia.org/wiki/File:Nickel_Nodule_obtained_by_Electroplating.jpg.
I'm wondering how feasible it would be to intentionally cultivate metal nodules like this, of a fairly large size (maybe 5 cm long?). My questions are:
1) How long does would this sort of thing take?
2) How serious of an endeavor would it be? Would I need massive amounts of equipment, extremely high voltage, etc? Or is this the kind of thing that I could do reasonably in a well-equipped art shop?
3) Is there any reason why it would be massively expensive?
- Austin, Texas
November 2, 2013
A. Well, quite honestly I don't think you would have much trouble with this idea. Essentially I would suggest a normal nickel plating bath and just run it for a really long time, might have to take a stab at this idea myself.
A simple Watts nickel bath should be fine; you should be able to get all the stuff together for under $100 if you're clever.
There are two things I suggest you watch out for:
One: most of your nickel substances are carcinogens, handle with care and read the warning labels. Adequate ventilation is also a necessity in this business.
Two: you're dealing with live current, please be careful and take appropriate safety measures.
"Standard" Watt's bath for reference:
Nickel sulphate, NiSO4.6H2O: 32-40 oz/gal (240-300 g/l)
Nickel chloride, NiCl2.6H2O: 4-12 oz/gal (30-90 g/l)
Boric acid, H3BO3: 4-6 oz/gal (30-45 g/l)
Blacksmith - Lenoir, North Carolina, USA
Copper Tree Electroforming
November 19, 2017Q. For the artist section of the board...
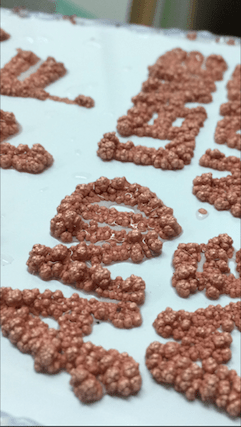
Hi there. This may prove to be a challenge, but I'm looking forward to your thoughts. I am an artist and have been experimenting with the use of copper plating and forming- my goal however is to produce large copper trees on various objects. Ive read some of the posts on this site about it -- but what has proved to be difficult is that most of the solutions and procedures described in my research here and elsewhere describes techniques to avoid treeing. Its hard for me to tell if proprietary baths for example will hurt or help my process. Ill lay out what I have for a set up now and maybe some people can let me know where I can improve. Im less concerned about the finish shininess for example, as I am about accelerating the process and maximizing the speed I can grow as I hope eventually to scale up to much larger pieces.
Unsure about proprietary solutions, and hoping to keep my costs down, Ive been making my own solution with copper sulphate
⇦ this on
eBay or
Amazon [affil links] at the rate of approx. 100-200 g/Liter. No adjuncts.
The anodes are copper pipes, or copper sheets hung in the solution at about 8" away from the receiving object or surface.

Currently I use a 30 amp DC power supply. I start with a very low current to get good adhesion and then I increase the voltage/current as the object increases in surface area.
Its very hard to calculate the surface area of the trees especially as they grow - so basically I have to wing it here. I'm currently running at 1 amp at 4.8 volts, but the form is rather large.
Agitation: I use a pretty massive home built plate spinner to keep the solution moving. I would consider a liquid or air pump if folks thought it was worth it.
Temperature: Its rather cold in my studio, especially at night easily between 40-60 °F on average. I do have the ability to heat the solution and maintain a temperature, but I wanted to keep things simple unless again, it would help accelerate the process.
---------
Indicators:
Burning seems obvious enough. If the voltage is too high, the trees are week and they collapse if you shake the plate or rinse it in water. Ill usually turn it down then. I do often get a red (Copper oxide) film on my anode. Im not sure how to interpret that, the cathode seems okay.
--------------
Questions:
1:How best do I dial in my solution for this process?
(Again, Im less concerned about brighteners as I am increasing the efficiency and time of growth. At some point - for example a large human sized object will likely require significant power- so I should start looking for efficiencies now.)
2:Is there a way to work backwards to calculate the surface area of the plating cathode (and therefore estimate my power needs) by say taking a resistance measurement of the solution?
Failing that: what other indicators can I look for to let me dial in the power.
3:I routinely take both the cathode and anode out of solution to rinse it off. Is that okay or can micro oxidation or something like that take place and possibly contaminate the surface of the growing form.
4: are there chemicals I should be considering to experiment with that would effect the treeing in various ways- say thicken up the dendrites, or increase them as they grow.
5: It seems common to include Sulfuric acid in many of these solutions- I have hydrochloric (1mole) acid around- could that be included instead
6: I'm not attached to copper, but it seems easy and affordable to experiment with. If this could be done easier in zinc, or tin for example, let me know and I can explore that path.
That's it for now. I love your site. Thank you so much. Happy to post my results if that's useful.
Best
Artist - Brooklyn, New York, USA
A. Hi Raphael
As in all art you will need to experiment, but for starters:
A "standard" copper sulphate bath is 150-200 g/l copper sulphate. You are correct that it needs acid -- 50-100 g/l sulfuric is common. I believe you can buy it as drain cleaner in US. Make certain it is sulfuric and always add acid to water; never the other way. Wear eye protection while mixing.
Most copper sulphate baths have a small amount of chloride in them but I would steer clear of hydrochloric acid for starters.
I used to make quite passable "Christmas trees" by starting with a bunch of bare copper wires in the approximate shape I wanted and build up from there. Just wind up the current until the tips start to 'burn' then back off a little.

Geoff Smith
Hampshire, England
November 22, 2017
Q. Thanks Geoff
I saw in some older guides a reference to a fully saturated copper sulfide^sulphate solution to which potassium alum is added - also to saturation. I have a fair amount of alum around -- can you see how it would affect the solution chemically other than increasing the relative saturation of the solution?
Secondly I've also seen references to using table salt rather than sulfuric acid as a secondary ingredient. That seems a little safer to have around.
Obviously I can trial these things heuristically -- but from a chemical point of view how do you see them acting on the copper itself.
Thanks again!
Raphael
- Brooklyn New York USA
November 24, 2017
November 25, 2017
A. Hi Raphael
Copper sulfides (there are several) are not soluble in water.
Did you mis-read sulphate?
I have no idea why alum is suggested and can think of nothing useful it would contribute. Common alum is potassium Aluminium Sulphate.
Table salt is sodium chloride. Chloride in the solution contributes to anode corrosion and has some effect on the deposit. It is only used in very small amounts and is unlikely to help which is why I suggested avoiding the hydrochloric acid which would have the same effect.
What you are short of is hydrogen ions. sulfuric acid adds this without adding anything you don't want.
I appreciate your reluctance to handle sulfuric acid. I wish more amateurs would recognise the dangers. If you can get hold of 'battery acid
⇦ this on
eBay
or
Amazon [affil links]
' that is about 50% sulfuric acid and much safer to handle.
If you can find a plating shop near you they may be persuaded to let you have some working solution but let them know you want it free from additives as these are specifically designed to prevent the effect you want.

Geoff Smith
Hampshire, England
Q. Thanks Geoff
I did mean "sulphate".
As an earlier commenter noted- I think a lot of this has to do with controlling the throwing power of the bath, assuming current flow is within normal parameters. I'll try and follow up with more pics if I can get these guys to tree up better. I'm still getting more of a layering effect than a treeing effect on the nodules. I'd like to encourage more dendritic growth and I see now why that chaos theory comment is very relevant. In a low throwing power bath the range of what gets plated (grows) vs what doesn't can be very small. As the object (cathode) gets larger or has parts of it a varying distances from the anode, very limited amounts of the cathode can be in play at the same time. I'm reluctant to rotate the object for conceptual reasons so I'll probably have to create a more sophisticated anode. Stay tuned. And thanks !!
- Brooklyn New York USA
December 15, 2017
Q, A, or Comment on THIS thread -or- Start a NEW Thread