-----
What could be causing the pitting and corroding to chrome kitchen stuff
Q. Can anybody tell me why my chrome curtain rails and lights in my kitchen are pitting & rusting slightly -- only seems to be the things high up.
All the things down low like handles don't appear to be affected.
I do have a flue-less gas fire in this room could that be the cause?
Thanks.
- Watford uk
February 23, 2020
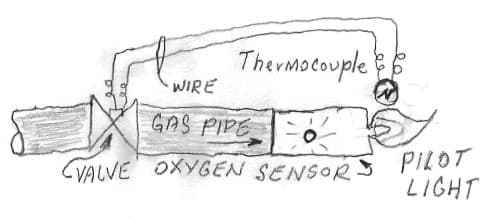
A. Hi Andy. I would not suspect that for two reasons: First, because I also have a ventless gas fireplace we use almost constantly and I don't have the problem myself. Second, because unless the UK is a lot different than the USA, ventless fireplaces have "oxygen sensors" that won't let them burn 'dirty'. Incomplete combustion or even too much dust in the air cuts off the flame. It's possible that it's sulfur in the fuel but I doubt it.
|
P.S.: As an engineer, always in search of 'the elegant solution', the 'oxygen sensor' on ventless fireplaces is the flabbergastingly simple ultimate one -- consisting of nothing but a tiny hole :-)
As people might know, the pilot light flame lands on a thermocouple, keeping it hot, which in turn keeps the gas valve energized to allow gas flow. If the pilot light goes out, the thermocouple cools, the valve closes, and gas won't flow through it until the pilot is relit and heats the thermocouple. |
What might be a more applicable explanation for your situation, is that plating -- most especially nickel-chrome plating -- ranges in quality from outstanding (able to withstand everything the world throws at it on a truck bumper for decades) to utterly abysmal (tiny rust pits while the items are still for sale). It is quite possible that your handles, designed for a bit of wear, have significantly better plating than your lights and curtain rails.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. It's your water. That's why it's only the faucet and not the handles. Water runs through the faucet so it's causing the pitting. I bet even if you haven't used the faucet for a while and the pitting is bad enough you can probably run your hand over it and it will be wet where water seeps through from inside the faucet. Like condensation. Mine are doing the same thing and I was told I have hard water. I'm on a well so I'd have to purchase a water softener to prevent this from happening. Hard water also corrodes pipes in the home causing water leaks. Which we have been having issues with now since our home is 25 yrs old. Hope this helped!
Angela McL- Raleigh, North Carolina
March 16, 2023
A. Thanks Angela. I don't think I agree with you at all, but I've been wrong before :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Corrosion all through my house
Q. I have corrosion all through my home; what could be causing this? I live in the desert so it's very dry here.
Debra Miller- Santa Clara, California
January 31, 2020
A. Hi Debra. With no description of exactly what's corroded, or pictures or history, sorry, it could be almost anything. Have you had the floors acid washed? Do you use a lot of straight bleach? Do you have strange smells from a neighbors's house. Give us some clues please :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. Debbie, You state that you live in the desert. Do you have a pool? Where do you store your pool chemicals? If you are storing them in an attached garage get them out of there.
Jim Wells- El Paso, Texas
May 13, 2020
Q. Hi Norm,
Since I use bleach to remove the stains on an old enamel sink, would a PVC drain pipe be better. My chrome pipes that are 14 years old are totally disintegrated and the mineral deposit made it necessary to remove the drain pipe with a hammer while the locking nuts on the p trap fell apart when I tried to turn them.
Hi Angela,
I am just now replacing my p trap and drain pipe and this is after 14 years. I have a water softener but the buildup around the pipes on the outside required a hammer to get it all out. Not sure if I should raise the number on the water softener but the water does not feel hard and does not make my hair feel unclean as hard water does. Nothing else is affected so I am wondering if using bleach to remove stains from my old enamel sink could be having an effect.
Homeowner - Interlaken, NY
October 4, 2024
⇩ Related postings, oldest first ⇩
2004
Q. Hello Norm,
Please I require some assistance. I work for a Kitchen and bath firm, that employs plumbers, builders, etc... My neighbor came to me for help. The chrome and all metals in their infant son's bathroom has become corroded, pitted, and/or rusted. The home was built 18 months ago. The builder is involved and took the chrome pop up from out of the sink to be tested. Per the builder, the test results state that a cleaning solvent had to have been slowly leaking in the vanity cabinet to have caused this. A bleach product. The pop up along with the faucet are of a brand name that I carry. I contacted the manufacturer who states that a vapor of sorts from bleach would not cause this on the product, and that you could use bleach on their product and it would not cause this. The shut off valves in the sink base, hinges on the cabinet the faucet and pop up outside of the cabinet the mirror above the cabinet, the light bar above and the light fixture in the hall way (side facing the bath only) all show the same signs.






The ironic thing is that the shut off valves to the toilet which is approximately 36" from the vanity cabinet do not show any signs. Humidity is not a factor as it is only used to bathe an infant. There is a heat vent located in the toebase of the cabinet that shows corrosion on the exterior of the vent plate but the interior is clean. Any ideas what could be causing this event to occur? I have pictures of all of this. I have thought perhaps something under the cabinet between floor and cabinet or something sealed in the wall. Please any assistance would be helpful.
Cara Magnusonbuilder/designer - White Lake , Michigan, USA
A. My experience -- although most of it is just handling letters on this site, rather than hands-on -- is that muriatic acid ⇦ on eBay or Amazon [affil link] or chlorine bleach is the culprit. Look for the use of this on the floor, or the wall grout, or even on the fixtures if the consumer is uneducated. Perhaps a toilet bowl cleaner with muriatic acid was used under the sink, or spilled, or was just left open. It's pretty hard to hurt good chrome with most things, but muriatic acid will do it in a snap.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Thank you for the response: We thought of Muriatic acid, But that was not in the area. And wouldn't you have to have used this often to get this effect? That would have meant that someone cleaned the interior of this cabinet with that? The lab report still states a chlorine or bleach slowly leaking. What would a vapor of sorts do over a long period of time? Could this be possible? What I am not sure.
Cara Magnuson- White Lake, Michigan
A. It would only have to be used on the floor or walls once and the fumes would drift through the bathroom destroying the chrome. But if it wasn't there, it wasn't there. But other cleaning products include muriatic acid. If bleach was used a few times I think it could attack the piping this way, although it looks like classic muriatic acid damage to me.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I have another question please, If we are thinking now that perhaps someone used muriatic acid to clean off excess grout, would it change the color of the grout? Perhaps make a white grout yellow?
Cara Magnuson- White Lake, Michigan, USA
2004
A. Sorry, I can't answer that one myself. However, I think it's acid attack ... and it seems more likely to be fumes than exposure to liquid ... which means I'd suspect an acid that fumes ... and muriatic acid does fume ... and muriatic acid is used for grout removal as well as in toilet bowl cleaners. I can't refute hard evidence to the contrary if it exists; but lacking any real evidence, and knowing that the chrome in that bathroom was exposed to something, muriatic acid fumes just seems more plausible to me than anything else; but wiping all that stuff with bleach is perhaps another possibility.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Ted,
I am the neighbor that Cara was referring to; the rust issue in a bathroom. Thank you for answering Cara's questions. I have a few more that I hope you could answer. We stopped using this bathroom 6 months ago. My 2-year old son previously used this bathroom to bathe, drink water, brush teeth, etc. Is this harmful/hazardous to small children? Is it an ongoing hazard? What needs to be replaced? Is it just the plumbing or the entire bathroom needs to be gutted? I know these are the 2 extremes, but I would honestly like to know what needs to be done. I appreciate your time with this matter. I've had many sleepless nights, thinking about it.
Thanks for all your help,
- White Lake, Michigan, USA
2004
A. Hi Melanie. Kids are tough, and encounter a lot of stuff; if he seems healthy I personally wouldn't give his health another thought. But, sorry, I have zero medical training and can't advise anyone else how they should feel or how they should react to their qualms.
You might ask the county health department if they have any sort of chlorine gas monitor to put in that cabinet for a few days. If that's not available, and there's no chlorine smell, I'd assume the chlorine was long gone and would replace the rusted hinges, cover plates, handles on the shut-off valves, and the drain pop-up arrangement because it's easy & relatively cheap, and it looks terrible.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. The question about the corrosion; some bleaches contain sodium hypochlorite (NaOCl) and this has a horrible smell of chlorine. If you have used a bleach that is based on hypochlorite, it could very well corrode as you have shown. Secondly, using muriatic acid (hydrochloric acid) seems a bit extreme to remove excess grout. Cannot you just scrape it off with a putty knife or "Scotchbrite"? Muriatic acid is a very strong acid and not pleasant to handle; it will readily attack most metals and cause corrosion when either a vapour or liquid. I know commercial cheap industrial hydrochloric acid (in the UK) can contain some iron and this will give it a slight green tinge, so if you put this onto grout, it may be adsorbed and turn the grout a pale green that will ultimately turn brown. However, pure hydrochloric acid should not contain anything that itself will turn grout a different colour.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
A. Trevor: I've done quite a bit of ceramic, porcelain, and quarry tiling. The grout is spread onto the tiles and sponged around until it mostly gets sqished into all the cracks/joints. This leaves a film of grout on the surface of the tile that you can remove while it's still damp by almost endless sponging & rinsing and dumping & refilling the wash bucket. That's the only way I've ever done it, but it is more time consuming than you could believe, and just when you think a section is done a corner of your sponge pulls some grout up from the joint and spreads it on the tile again :-) Once the grout is dry you can't remove it that way, and your only option is acid washing to dissolve it.
Contractors apparently sometimes can't afford the time for the endless washing & rewashing & rewashing & rewashing ... and do just a quick wipe up, and then go back and remove the grout haze from the tile with acid. If the acid is too strong or they don't have excellent ventilation, or don't use great care and have an empty house, the fumes are likely to corrode stuff.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. My wife is convinced that using household laundry bleach (Clorox brand) cleans rather than damages what appears to be the chrome-plating on the fixtures of our apartment's sinks. She is convinced that the greenish pitting and other discoloration is due to a lack of cleaning. It was clean and polished when we moved in and only became pitted and greenish stained days after we moved in and she 'disinfected and cleaned' using household laundry bleach. She was convinced that some past turbidity of our drinking water and poured much bleach into our downstairs sink drain especially and a black-stained powder-like matter to then settled along the sides of the closed sink also filled with water as I understand it. (I'd give you more details, but my wife is convinced that bleach doesn't act that way toward some or all metals.) H-E-L-P, before her actions result in our having to buy all new plumbing fixtures.
Mike Smith- Portsmouth, New Hampshire, U.S.A.
March 1, 2008
A. Mike: Bleach is a great disinfectant so I understand why your wife would use it, but it's terribly corrosive to metal for the same reason that it kills germs: tremendous oxidation power. If you search the site there are a lot of student science projects where the kids are looking for what liquid will cause the most rust fastest and they are usually amazed at how quickly bleach corrodes metals. A good new nickel-chromium plating may hold up for a short while, but once there are some wear spots or pinholes the bleach will be incredibly corrosive.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I have a question about chlorine in water, I have dezincification resistant ball valves installed in a potable water system with brass chrome plated balls, the ball were nickel-plated before being chromed. The potable water system splits to serve the cold water and hot water systems. The potable water system has a chlorine injection system controlled to maintain 0.5 ppm to control bacteria in the system. I was called back to site to investigate why some of the ball valves on the hot water circuit have jammed but not on the cold. On investigation I found that the chrome plating on the balls had started to flake and the nickel-plating underneath was showing signs corrosion.
I have since found out that chlorine in hot water is more aggressive by a factor of 2 for every 10 °C rise in temperature, the supply temperature is 65 °C, but I can find no documented evidence that chlorine is known to attack chrome or the nickel-plating when used in hot water.
Any Help?
By the way what I do know is that if fumes from a chlorine type product are left in as unventilated area, such as the cupboard under a sink, the fumes will attack any material that is plated such as chrome and galvanise.
Valve Manufacture - London, UK
February 6, 2009
Ed. note: I'm sure that Cara & Melanie appreciate your confirmation that open bottles of chlorine-rich cleaners in a closed cabinet will corrode the metal in it.
Q. I have been wondering why my bathroom's (which has only a vent and no window) chrome fixtures were constantly corroding - I use lime away to scrub the chrome clean -I also use bleach to kill the mildew created by this poorly designed bathroom. Am I to understand that if I discontinue to use the bleach, my problem will be solved? What cleaning products will not cause corrosion? Shall I use baking soda or something instead? Thanks for your valuable input!
Linda Tanghe- Webster, New York
March 24, 2012
A. Chlorine bleach can indeed cause these types of issues.
It can happen even faster if it's used in a strong solution.
Very dilute solutions are not as harmful but can, over time, also cause damage to chrome plating.
Toilet bowl cleaners can also attack chrome fixtures especially acid bowl cleaners commonly used by professional cleaners.
Green oxides, black corrosion and stripping of the chrome plating partially or entirely are the result.
Home improvement contractor - Orlando, Florida
November 15, 2012
A. I routinely use bleach to remove chrome plating from plastic parts. I just immerse the parts and wait a few weeks. The chrome turns into a black sludge in the bottom of the container. Does no one do experiments anymore to investigate a hypothesis? Check out www.sciencedirect.com/science/article/pii/S0261306906002469
Stephen Chapman- Greensboro, North Carolina, USA
Q. I have a new faucet issue since my original post and pictures above (Cara Magnuson). I have since moved to Arizona, remarried, and had a home built. The brushed nickel faucet has gone brassy only on the top part, and the cultured marble counter top has discolored to yellow on the surface part to the right side.



The color change started approximately 1-1 1/2 years after the home was complete, and only to the guest bath that is rarely used. The faucets in the other baths are the same and no discoloration is occurring to them. Any thoughts on what's happening with this?
builder/designer - Sun City, Arizona, USA
January 22, 2014
A. I would guess that the faucet in your guest room has more undisturbed time to form oxides than the others in your house.
Just a guess, but it seems possible.
Blacksmith - Lenoir, North Carolina USA
February 28, 2014

The last pic above seems to have been clipped and the discoloration of top is not showing; here is a cropped area of the above far right photo...
Q. That is possible BUT, I would think it would be more uniform, not just the the top of the faucet and the right side of the counter top...
My husband uses that bath on occasion and guests. But is not used as constantly as the master bath that has same faucet and top ... the only difference in the two baths is the master bath does have a small window to allow natural light in BUT, again with the non-uniformity of the discoloration I'm not sure that would affect it ... if the whole faucet and top discolored I could see some significance to natural light and usage. But it has me at a loss to the right side only on the cultured marble, and the top of the faucet, not the handles etc. They are both cleaned the same day with same cleaners... just at a huge loss for why it is and what is causing it...
builder/designer - Sun City, Arizona
March 1, 2014
A. The cause of the fixture is definitely muriatic (aka hydrochloric) acid. It is the primary ingredient in toilet bowl cleaners. Why? Because toilet bowls have no metal in their assembly. Hydrochloric acid (HCl) slowly removes chromium plate. It may take a year or two to see the green crusty stuff. That is the underlying copper contained in the chrome plated brass parts. Eventually, the removal of the chrome reveals the gold-colored brass which corrodes quickly when exposed to HCl, creating more green stuff. Toilet bowl cleaner may not be there now, but it could have done the damage months ago. Don't doubt this. Look at the ingredients in toilet bowl cleaner. The key letters are HCl. This problem is prevalent throughout the world but is not understood. Walk into any restroom that has been in existence for a few years. It's everywhere.
Richard Roberts- Brooklyn, Wisconsin
December 6, 2014
Q. I moved into my new apartment May 10th. Today I noticed that my shower caddy is all rusting, as are the new shower curtain hooks. I have never experienced this before. What is causing this and how can I correct the problem.
koren cristman- flemington, New Jersey
June 29, 2016
A. Hi Koren. Flemington NJ doesn't sound like an especially corrosive place. Have you used bleach or muriatic acid in the bathroom? If not, I'd say your caddy and shower curtain hooks are cheap Chinese garbage. Unfortunately, nickel-chrome plating which is not done well is worse that bare steel with no plating at all because, if there is any porosity or pinholes, the underlying steel is forced to become a sacrificial anode protecting the nickel plating. Please see our FAQ, "Understanding Chrome Plating" for both an understanding of this, and a close-up picture of a crappy shower caddy rusted before the big box store even sold it :-(
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
A. I've worked in the hotel industry for more than 22 years in the housekeeping department. The oxidation you see on all metal fixtures is due to chlorine vapors. Chlorine reacts to organic materials such as lime, or even the air we breathe. The strong smell is not just a chlorine smell, it will deteriorate all metals and that's because chlorine is an oxidizing agent. Make sure not to keep bleach bottles inside cabinets. Make sure to rinse throughly after use. And only use it to remove stains. For disinfecting bathrooms use other agents such as quaternary ammonium, or a peroxide based disinfectant. Hope this helps.
L mora- Punta Cana, Dominican Republic
Q. A full bottle of toilet bowl cleaner spilled under our bathroom sink and we did not know it until all metals in the bathroom began to corrode. The mirrors in the vanity began to fog. I'd wipe them and they re-fog. 2 rooms away metals in my jewelry box are corroded. We have removed the bottom of the cabinet and taped it off waiting on a new vanity. There must still be remaining cleaner because metals are still corroding and the mirrors continue to fog. I've had asthma like respiratory symptoms and continue to. Just returning from two weeks away and being in the room I can smell it. What will neutralize this chemical? Are we putting ourselves in harm's way?
Dj Farber- West Bloomfield, Michigan USA
February 28, 2018
A. Hi. I can't advise on medical issues, but the chlorine fumes will eventually dissipate -- the more ventilation you can provide, the sooner. If weather permits, or if you have another bathroom, keep the window wide open, any vent fan on, and maybe a portable fan inside the cabinet. baking soda [in bulk on
eBay
or
Amazon [affil link]
will help, but you also want excellent air flow.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q, A, or Comment on THIS thread -or- Start a NEW Thread