
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Problem with Electrocleaning of Brass
Q. We have trouble with a Raw Material (70-30 Brass), possibly it was covered with a antirust oil. After electroless nickel process, the product show stains and is not brilliant (although we use an additive for that). We use an electrolytic cleaner and hydrochloric acid. What kind of degrease method can we use?
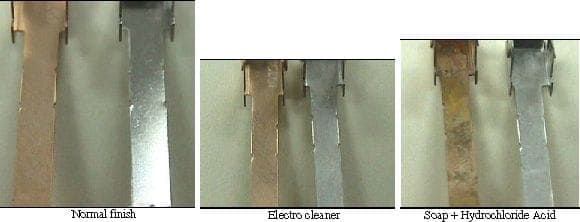
- Tijuana BC, México
2003
A. You should do a water break test after the cleaning and acid cycle. If you still have water breaks, some oil or organic compound might be present which was not totally removed by your electrocleaner, so you probably need to add a preclean step by means of a vapor degrease w/approved solvent.
Guillermo MarrufoMonterrey, NL, Mexico
2003
A. You should be using a soak & electro cleaner designed for brass. Cleaners for steel will tend to pull carbon out of the brass (too brutal). Acid will not remove it. Do not over-clean brass. Some experimentation may be required. Many times you can have no current on the electro cleaner and still run the parts through it. Contact a proprietary manufacturer. Many support this web site.
William F. Hemp- Grand Rapids, Michigan
2003
Q. Dear Sir,
I am Ahsan From Pakistan. I used cleaning from 3 suppliers, but the waterbreak problem not Solved. Kindly suggest solution for water break.
OMER Brothers & Co. - Lahore,Pakistan
June 27, 2019
Ed. note: This RFQ is outdated, but technical replies are welcome, and readers are encouraged to post their own RFQs. But no public commercial suggestions please ( huh? why?).
A. Hi Ahsan. Can we assume that you did electrocleaning with those three different chemicals and they were all designed for electrocleaning brass? There is nothing unusual that you are not mentioning, like deep recesses or burned on carbon deposits? The electrocleaning tank is not the initial step? You are doing something first like soak cleaning, ultrasonic cleaning, power washing, solvent cleaning, etc., so you are not overloading the tank with grease?
Thousands of shops successfully electroclean brass, so we need to know what is different about your situation. Remember that grease floats to the top of the tank and the parts therefore exit the tank with a coating of oil on them if the tank is not kept clean. Thanks!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
June 2019
Q, A, or Comment on THIS thread -or- Start a NEW Thread