
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Solutions to Ebonol C blackening problems
Q. Hello,
We are looking for a wax that was supplied to us in the past (over 20 years ago) from Enthone - the Liquid wax was ENTEK WAX-18. We used this wax to finish Brass Eyelets after the Black Oxy process. We are currently speaking with MacDermid who say it's no longer available and they are looking for an alternative. Can anyone suggest a wax to use in the finishing of Black Oxy, or is ENTEK WAX-18 now sold under a different name? Any and all help appreciated. Thanks.
- Barcelona, Spain
January 31, 2025
A. Hi Niamh
I can't find a safety data sheet for that product but I think it's a fair bet that it has been discontinued because it included trichlorethylene :-(
We usually don't print brand names or sourcing info time has proven it often creates a mess ( huh? why?), but it may be unavoidable in this case. We do ask readers to try to stress the generic technology in any responses rather than starting a race to the bottom with responses of the style "my product is better than theirs" 🙂
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
⇩ Related postings, oldest first ⇩
Blackening Candlesticks
Q. I want to change some brass candlestick holders from brass to black without ruining them.
Gill Manning- Hertfordshire, England
2002
A. There are two basic options. Both involve making sure the brass is very clean before you start or you will have problems with adhesion and good film thickness. I suggest you give the brass a very good clean in both acid and alkaline cleaners; the exact process will depend on what you have available. You can then paint the brass with a suitable paint; probably acrylic is the best option, although if you have access to electrophoretic paint, this is better as it will get into all nooks and crannies and will give a more consistent coating.
Secondly you can dip the brass into a sulfur solution. This is usually made by dissolving flowers of sulfur in a solution of sodium sulfide. The solution will go a sort of yellow colour and will stink, but it is very good at blackening metals. However, its success may be dependent on the type of brass you are using as different brasses have different amounts of zinc in them.
To get best consistency, try carefully etching out the brass from the surface, but be careful, as removing too much zinc will result in "dezincification" which will leave a copper sponge with no structural strength.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Multiple threads merged: please forgive chronology errors and repetition 🙂
Blackening Jewelry
Q. Hello,
Our company manufactures promotional and recognition award jewelry. We have been using a product called Ebonol C in a bath solution for blackening brass (85/15, copper/alloy) emblematic jewelry (tie tack and lapel pins mostly).
The parts are blackened and then buffed on an semi aggressive lea wheel to achieve an antique finish look. The parts look great at this point. We then mask off certain areas with ochre and buff them again with high gloss polish wheels. We then wash off the ochre and polishing compound in an aqueous degreaser and ultra sonic. This process has served us well until lately. Now the black ox is falling out in during the washing process no matter how carefully guarded. Is there a trick to washing parts that have been oxidized with Ebonol C? Or is there another blackening agent that might be better suited to our needs?
Any input would be greatly appreciated.
Sincerely,
Roland Dion- Attleboro, Massachusetts
2003
A. Ebonol C is the world's standard for applying black oxide coatings on copper and its alloys such as brass. Nearly everyone in the finishing world has done it at one point in their career. There hasn't been any change in the formulation of the material, to my knowledge and on going experience, and while the manufacturer's name has undergone change after change due to acquisition, it's the same old Ebonol C.
Couple of thoughts: if the Ebonol hasn't changed and your process for applying it hasn't changed, look at what happens ahead of Ebonol application. Specifically, what buffing compound changes have been made. Also, what about your brass - has the lead content or other alloying elements changed? In order to get the best black on brass, sometimes need special "Brass Activator."
As one who owns a shop that has done Ebonol C for nearly 40 years and still gets out and dips his hands in the "vats", better look elsewhere. Our Ebonol is still working fine.

Milt Stevenson, Jr.
Syracuse, New York
A. Check your acid activator.
Russell Richter- Danbury, Connecticut, USA
A. I use a chlorine solution to blacken sterling silver and I also use iodine [affil links], like you buy for cuts, to blacken gold. Try one of those to blacken the brass.
John Legere- Lake Worth, Florida
2007
Blackening Brass as Black as Possible
Q. Hi All,
Looking for the most effective way to give a brass component blackest finish possible.
I have the following reference I'm trying to match. My component is very similar, a brass machined rod 63.5 mm x 225 mm.
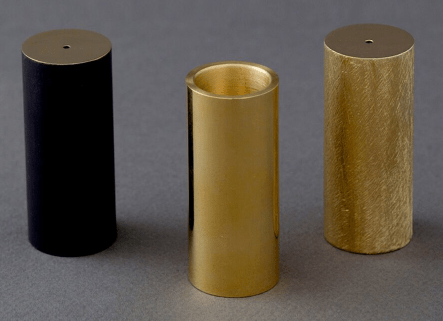
I know this about the above: "Each object is hand made under the supervision of Carl Aubock IV at the Carl Aubock workshop in Vienna, founded in 1900"
All/any expertise very much appreciated.
Cheers,
Designer - London, UK
August 20, 2015
A. Hi Hayden. Selenium dioxide is what trophy suppliers use to get the dark black lettering on trophies. Ebonol C is the best known commercial blackening process for brass.
Sorry, we can't print any comments about what Carl Aubock IV specifically might or might not do -- that would be a bit too close to crowd-sourcing industrial espionage :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. What Ted refers to is also the chemical bath that faucet companies use to create an oil rubbed bronze patina on brass. It results in a black finish if you don't do it right. I know this for a fact since we just went through the process of matching an oil rubbed bronze patina with a slightly brown tinge. The bath is an acidic solution containing selenium, and is commercially available.

Jim Treglio - scwineryreview.com
PVD Consultant & Wine Lover
San Diego, California
A. There's another, completely different brass blackening process. I do not have the formula in front of me, but you'll find it in the OLD "Metal Finishing Guidebook". ⇦ this on eBay or Amazon [affil links] is dissolved in full strength aqua ammonia, and the parts immersed in it at room temperature. A shop I used to work for used this method on some brass cylinders they got once in awhile and it seemed to work fine.

Dave Wichern
Consultant - The Bronx, New York
Readers may also be interested in these related threads:
• Topic #32355 "Black Oxide on Brass / Black Anodizing of Brass"
• Topic #1198 "How to Darken Copper, Brass or Bronze"
Q, A, or Comment on THIS thread -or- Start a NEW Thread
