
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

Thread 172/03
What Acids are in fruit juice?
Q. Hi, My son is doing a science fair project and he is wondering what in the lemon and tomato juice is removing the tarnish and rust from the penny? He knows that it is an acid but is there any specific kind of acid? Also is there any other good reference web sites you can give me for him to go to?
Thank you so much
Brandon J. [last name deleted for privacy by Editor]- Baltimore, Maryland
2002
publicly reply to Brandon J.
Ed. note: Please see our FAQ on Student Cleaning of Pennies
A. Brandon,
Lemon juice is saturated with citric acid . Tomatoes also contain both citric and folic acids. These naturally occurring acids remove tarnish and corrosion from metal surfaces due to the strong positive charge of hydrogen in the acids. The "cationic" hydrogen, along with water moisture, changes (raises) the tarnished coin surface metal to it's ionic (molecular) state; making it dissolve into liquid very easily.
One more thing you might want to tell your son: The term "rust" refers only to the corrosive state of iron. When other metals oxidize, their tarnishing is called corrosion. Pennies are made of copper coated zinc, so they don't "rust".
Any other websites? How dare you? ~8)

Randall Fowler - Fowler Industrial Plating, LLC
Cleveland, Tennessee, USA
2002
publicly reply to Randall Fowler
A. Lemons contain a lot of citric acid , hence they are known as "citrus fruits" (along with limes and oranges). Tomatoes contain some ascorbic acid (Vitamin C) which is an anti-oxidant and because of this some people believe tomatoes stave off the chance of cancers. Tomatoes also contain malic acid and oxalic acid ( also found in rhubarb); it also contains folic acid, which is useful if you are pregnant! Many fruits contain sugars and when sugars ferment they turn the sugar into alcohol, which in turn can be turned into acetic acid ⇦ this on eBay or Amazon [affil links] - tomatoes are not very rich in sugars, so the acetic acid level will not be very high. Believe it or not, lemons can contain a fair amount of sugar, so potentially a well ripened one could contain acetic acid . All of these acids will clean old pennies given enough time.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
2002
publicly reply to Trevor Crichton
Q. How much citric acid is in apple juice, orange juice, lime juice, or lemon juice?
Ashley H. [last name deleted for privacy by Editor]- Midland, Texas
2003
publicly reply to Ashley H.
Q. I need help with my project: which has the strongest acid orange juice apple juice or grapefruit juice I NEED THIS BY THURSDAY OR I'LL FLUNK AND THE ORKS IN MIDDLE EARTH WILL GET ME.
Brian D. [last name deleted for privacy by Editor]- Buffalo, New York
2004
publicly reply to Brian D.
A. The orange juice has the most acid over all of lemon juice and whatever the other one was!
Dedra K. [last name deleted for privacy by Editor]- Altair, Texas
2004
publicly reply to Dedra K.
A. Do you have a reference you consulted, or an experiment you ran, Dedra -- or was that just a guess? My guess is that you are incorrect and that the right answer is lemon juice. My guess is based on the fact that acids taste sour, and lemon juice tastes more sour to me than orange juice.
The way to actually find out is to test it with pH paper.
Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
publicly reply to Ted Mooney
Q. I am doing the same project...what a small world...but anyway...I am using carrot juice along w/lemon and tomato...so if anyone knows the acids in carrot juice..that would be a great help!
Thanks so much!
Lauren M. [last name deleted for privacy by Editor]- Little Rock, Arkansas
2004
publicly reply to Lauren M.
Q. HI MY NAME IS JAY, I AM ALSO DOING THIS SCIENCE FAIR PROJECT AT MY SCHOOL AND I NEED SOME HELP. THE JUICES I HAVE WRITTEN DOWN ARE LEMON JUICE, LIME JUICE, ORANGE JUICE, PINEAPPLE JUICE, AND GRAPEFRUIT JUICE. I THINK ALL OF THEM HAVE ACID IN THEM BUT I AM NOT SURE. BUT WHAT I REALLY NEED TO FIND OUT IS WHICH ONE OF THOSE JUICES HAS THE MOST ACID. WHEN I FIND THAT OUT MY PROJECT WILL BE MUCH EASIER. SO IF ANYONE HAS THE ANSWER TO MY QUESTION I WILL REALLY APPRECIATE IT. THANK YOU FOR YOUR TIME.
Jaeshaud B. [last name deleted for privacy by Editor]- Antelope, California
2005
publicly reply to Jaeshaud B.
Q. Hey, I'm in my last year of high school (yr 13) in New Zealand, and as part of our chemistry course, we must carry out an investigation task. I have chosen to test using titrations to see how much citric acid is in orange juice and would appreciate any information or links to help me out!
Thanks,
student - Hamilton, New Zealand
2005
publicly reply to Tracey F.
by Steve Spangler
on AbeBooks
or eBay
or Amazon
(affil links)
Q. I am doing a science fair project and have cleaned pennies in orange, pear, blackcurrant and mango juice. My findings are that the pear juice was most effective, then mango, then orange and then blackcurrant. I am wondering why this would be? One web site suggested that pear juice has a lower acid content than other juices - is it the acid content that cleans the oxide from the pennies or a different product in the juice?
Nathan C [last name deleted for privacy by Editor]Student - Palmerston North, New Zealand
2006
publicly reply to Nathan C
A. Hi, Nathan. I feel your pain. Juices are complex mixtures of a hundred or a thousand chemicals, of variable concentration depending on country of origin, ripeness, and other factors -- and it might be wrong for you to try to attribute their cleaning power to acidity alone. Many factors can be involved -- chelation by citric acid s, complexing by tartaric acid ⇦ this on eBay or Amazon [affil links] and the several other acids mentioned by Mr. Crichton, fermentation to alcohol, etc.
Please ask your science teacher for pH paper or a pH meter and try to relate the cleaning ability to the pH of the juice (the acidity of the juice). You will find, though, that you can easily disprove the relationship simply by adding a pinch of salt, which fabulously improves the tarnish removal capability of mild acids like fruit juice.
Best would be to use this project as a chance to practice good lab method. Keep a "lab book" -- this is a notebook where you number the pages in advance of writing anything else so you can't tear pages out and pretend some observation which you made never happened :-)
Then you write down everything you do and everything you see, in ink, with dates and times. The important thing in science is you don't decide in advance what the results of the experiment should be and then mess around until you get them -- that's a poison called "junk science". In real science you immediately write down what you see! This properly done lab book should earn you an "A" whether or not you can fully explain the reason behind the results you got. Good luck, Nathan!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2006
publicly reply to Ted Mooney
A. I too am doing the same project, look on the US government website under the USDA and they have a breakdown of how much acid is in each different type of orange juice. It sure helped me!
Mindy C. [last name deleted for privacy by Editor]- Port Orange, Florida
2007
publicly reply to Mindy C.
Q. MY SON HAS A SCIENCE PROJECT AND HE NEEDS TO KNOW WHAT FRUIT JUICE HAS THE MOST ACID. ALSO NEEDS TO KNOW THE AMOUNT OF ACID IN EACH ONE. THE JUICES ARE APPLE, ORANGE, GRAPE AND LEMON. THANKS FOR YOUR HELP
DARLA W [last name deleted for privacy by Editor]- BLEDSOE, TENNESSEE
2007
publicly reply to DARLA W
A. Please try the USDA site that Mindy recommended, Darla, and let us know what you find there and what you need that it lacks. We'd appreciate the feedback. Thanks!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2007
publicly reply to Ted Mooney
! Here is an Internet phenomenon that I had not thought of: using it to avoid doing a science project.
Kids (and even worse, parents) who are trying to get the answers to what is actually an excellent science project: Do some work! Get the pH paper suggested in one post, and figure it out yourself! You might even find that you have fun!
Posters who are trying to help: STOP! Think about what you are doing -- this is just one of many reasons that kids in the US perform so dismally compared to their peers around the world in science!
- Heidelberg, Germany
January 23, 2008
publicly reply to Gary Cregan
I mostly agree with you, Gary. But on those occasions when a student is poorly prepared for what s/he is going to be doing, and we can see that s/he will be reinforcing junk science instead of learning the scientific method, and is on course to learning to despise science instead of embracing it, I think we should try to steer them back on track.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
January 25, 2008
publicly reply to Ted Mooney
! To all those doing the final year Chemistry assignment with acids, titration and juice, SO AM I!
My group is doing orange juice and we need to find the acidity level using titration We're also doing a titration method for ascorbic acid and using density to find sugar.
Anyways, guys, jump on the internet. It's boring but you get your answers yourself.
- Brisbane, Queensland, Australia
March 14, 2008
publicly reply to Sasha P
July 14, 2008
Q. I'm a +2 student. I know that the acid present in tomatoes is oxalic acid and that in lemon juice is citric acid . I also found that the acid present in banana is butyric acid. From this my curiosity starts and I'm now doing a project, i.e., I'm now collecting about the acids present in different vegetables and fruit juices. I want to submit this project to my sir who is my guide and a good advisor of mine, by next Monday. So, please Help me this is my first request to you. Hope that you will not disappoint me. THE DIFFERENT FRUIT JUICES I WANT TO KNOW IS AS FOLLOWS
ORANGE JUICE
APPLE JUICE
GRAPE JUICE
CARROT JUICE
PINEAPPLE JUICE
MUSSAMBI JUICE
student - Kochi, Kerala, India
publicly reply to Athulya jyothi
Q. I have a science project do and I need to know how much acid is in lemon juice, I am in the 7th grade.
Kaleena D [last name deleted for privacy by Editor]student - Margate, Florida
October 14, 2008
publicly reply to Kaleena D
A. Hi Kaleena. I suspect that you don't really have a good understanding of what you mean by "how much acid is in ...?". You might actually be interested in the pH (which is NOT the amount of acid) or the acidity as determined by a neutralization/titration with alkali, or a percentage of citric acid or ascorbic acid, etc., etc.
If you seek an answer to a question without really understanding the question, you're soon stuck in a quagmire. Please try to have a dialog with your teacher until you very clearly understand the question you are trying to find the answer to. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
publicly reply to Ted Mooney
December 3, 2008
![]() Dear All Students and Posters of This Topic:
Dear All Students and Posters of This Topic:
The purpose of science projects is to enable both the conductor of the experiment as well as the spectators to LEARN some cool things about chemistry.
The heart of science is achieving progress through research, experimentation, and honestly, large amounts of dedication and hard work.
Parents and students- STOP asking questions in hopes of getting answers. Use that time instead to teach or learn something about research methods. Laziness has very rarely helped the progress of science or mankind in general. Also, if you are taking answers off of this website instead of doing the research and experimentation, MAKE SURE YOU ARE CITING THIS WEBSITE AND FORUM AS A REFERENCE. If you don't, that is PLAGIARISM.
I highly doubt any science fair judge with half a brain would look kindly on a project getting most of its data from an internet forum.
Parents, BE vigilant and for Christ's sake, do the RIGHT thing and teach your kids how to work hard for what they seek. All I have seen from every parent in this forum is a parent teaching their kids to get answers off the internet without citing the sources (plagiarism and LAZINESS). Do your job as parents.
Regardless of how the US's educational system may or may not be equipping our youth with proper education, research technique, or resources, their are MILLIONS of sources to consult for the answers you seek. Keep in mind that a large majority of our most talented scientists in the world grew up in Third World countries, war-torn countries, during the Great Depression, etc. They had it much worse but still managed to pull off some of the greatest findings in science.
Stop blaming the educational system (although there are faults...lots of them), and take accountability for your own education!
- Boulder, Colorado
publicly reply to Charles Oh
Q. Well, I AM interested in helping my daughter grasp the scientific process. Her assignment is to predict which fruit juices will change in acidity over time. I am not sure what theory this is based on that pH changes over time. She knows she can't make any predictions until she has some background knowledge. Any suggestions about how to research this variable would be greatly appreciated!
Ellen Carpenter- Norfolk, Virginia
March 25, 2009
publicly reply to Ellen Carpenter
A. Hi, Ellen. I appreciate your input on this, but personally, I wouldn't do it that way. Rather, I'd pick a practical number of fruit juices of various sorts, and measure the pH before and after the elapse of some time, and THEN try to research a reason for what she finds --
First, your daughter will enjoy it far more because she will be charting new territory with you where mom doesn't already know the answers, rather than trying to keep up with mom's knowledge, which will prove boring for her.
Second, there will be no "junk science" -- trying to talk yourself into seeing things you may not be seeing, and writing off observations as random flaws, as you subconsciously fudge results to try to match notions you think you learned in the research.
Discovering a phenomenon and trying to uncover the reason for what you have discovered is much more fun, far more rewarding, and a lot better science than trying to concoct an experiment to prove somebody else's theory. And it's faster and easier because after you've done the experiment you will know precisely what you are trying to research, rather than just having just some vague notion that there must be something out there that accounts for why different juices may or may not change pH quicker than others.
After you've run the experiment, and then done research on your specific findings to try to account for them, THEN you might run the test again to try to prove that theory, but don't even dream of discarding the results from the first run. Best of luck!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
June 2009
publicly reply to Ted Mooney
Q. My name is Carly an I am doing a science project. I am in fifth grade and my science grades and expectations are not that well. I would like to know by this Friday. Here is my question.."does fruit conduct more electricity than vegetables?". For one, pickles is my first vegetable and I don't know any other veggies with acids in them at this point in science. I would be so glad to hear a result and I will try to find more about this now! PLEASE HELP ME ANYBODY!
THANK YOU,
CARLY:)
P.S: I WOULD LIKE THIS BECAUSE FINALLY IN SCIENCE I WILL GET AN "A" SINCE SECOND GRADE. THANK YOU SO MUCH I APPRECIATE IT :)
I am a student - chuluota, Florida
October 14, 2009
publicly reply to Carly W
A. Hi, Carly. I hate to break it to you, but a pickle is not really a vegetable :-)
It is a cucumber which has been steeped for months in very strong saltwater to replace its natural liquids with this strong salt brine. So its conductivity is owing to the salt brine, not its vegetable nature.
But pick a few fresh vegetables like cucumbers, radishes, potatoes, onions and turnips and see what you get :-)
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
October 16, 2009
publicly reply to Ted Mooney
Q. Hi, I need serious help in setting up an experiment to find out if green skinned fruits are more sour than other coloured fruits. So far I know that I would get a green skin fruit (green skin orange and a yellow skin one), I would juice it. and I think use a pH paper to test the acidity, but do I just place the pH in the juice.
I'm in grade 10
I await for your response, thanks in advance.
student - St. Mary, Jamaica
February 17, 2010
publicly reply to Tashi Tait
A. Hi, Tashi. Yes, just put the pH paper in the juice, pull it out, and see what color it is, and what pH that indicates.
But please don't "await" a response; this is just a public forum, not a homework hotline :-)
Luck and Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
February 18, 2010
publicly reply to Ted Mooney
Q. I also have the same lab to take in on Monday, I had that same idea -- of the whole pH paper thing. It's a PD lab, meaning I have to plan an experiment from home. I don't have any pH paper or universal indicator paper and I need to know the acidity of at least 3 fruits. Please help!
Georgia B [last name deleted for privacy by Editor]- St. James, Jamaica
March 13, 2010
publicly reply to Georgia B
A. Hi, Georgia. I'm not sure what you want help with. You are wanting us to help you by urging you yet again to get pH paper from the teacher? Or are you asking us to take a wild guess at the pH of your particular three pieces of green fruit, and another wild guess of what the pH would be if you picked fruit that was no longer green?
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March , 2010
publicly reply to Ted Mooney
Q. Hey, I'm in my last year of high school (yr 10) in Australia, and as part of our chemistry course, we must carry out an investigation task. I have chosen to test using titrations to see how much citric acid is in different fruits, and one of the main questions are: does the acidity of fruit change with age? I have looked everywhere and have found no information in this area and I REALLY, REALLY need some help!
Thanks so much!
student - Hobart, Tasmania, Australia
April 15, 2010
publicly reply to Brigid N
A. Hi, Brigid. Sorry, but I really don't understand your quandary at all. You say you are going to do titrations. So, do the titrations with a fresh fruit and do them again when the fruit or the juice is older. What is the complication? You certainly don't want to know the answer to shoot for before you do the titration because that would lead to "junk science" (discounting contrary results, giving too much weight to agreeable results, re-doing the experiment until random factors make it turn out the way you want).
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
April 15, 2010
publicly reply to Ted Mooney
![]() I just wanted to say thank you for the information you provide in this forum and the conscientious way you post it. You give some info but then expect the reader to dig further. Thank you for helping these kids while at the same time challenging them to be better!
I just wanted to say thank you for the information you provide in this forum and the conscientious way you post it. You give some info but then expect the reader to dig further. Thank you for helping these kids while at the same time challenging them to be better!
Dad of a 7th grade science fair participant!
Dad of a 7th grade science fair participant! - San Antonio, Texas
February 21, 2011
publicly reply to Shane McIntosh
Q. How to determine acidity level in different samples of fruit and vegetable juices?
Hi, I am Vipul a high school student from Delhi, India. I am preparing a project on different experiments to determine acidity level in different samples of fruit and vegetable juices. I am not sure what and which experiment should I go with; please help me with it.
Vipul M. [last name deleted for privacy by Editor]Student - New Delhi, India
June 14, 2011
publicly reply to Vipul M.
Q. I have to do a pd to determine if green skinned fruits are more sour than the others/
nickacy h [last name deleted for privacy by Editor]- georgetown, guyana
April 8, 2012
publicly reply to nickacy h
A. C'mon students :-)
pH paper is the dead easy way to determine acidity. Until you've mastered pH paper, you're not ready to move on to trickier methods like titration.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
April 9, 2012
publicly reply to Ted Mooney
Q. Hi, I'm doing 2 p and d's and need help please. The first one its hypothesis is "green skinned fruits are more sour than coloured skinned fruits". Now the problem is that my teacher said she doesn't want anything like using litmus paper, pH, acidity, juicing or tasting so I'm left to wonder what experiment I could do.
And the second one is salt slows down the rate at which ice melts. Could you give me any ideas for both please. Thank you in advance :)
Diana W [last name deleted for privacy by Editor]- Dominica
June 6, 2012
_[last_name_deleted_for_privacy_by_Editor]" rel="noopener nofollow" target="_blank">publicly reply to Diana W [last name deleted for privacy by Editor]
A. Hi Diana.
You obviously misunderstood your teacher's instructions for the first experiment and already realize that it makes no sense. Don't compound the problem by asking people to guess what your teacher said; ask him/her what was meant.
For the second experiment you need to get ice, salt, and a clock or other timing device. Put measured amounts of ice into two similar containers, and pour a measured amount of salt into one of them, stir it up and carefully record what you see happening. One thing your assignment doesn't mention, but might prove interesting to employ, is a thermometer. You might record the temperature in the two buckets as the ice melts.
There is also one little distortion your experiment introduces. The salt, if room temperature, will warm the ice you put it into. And if the salt is ice cold, then you'll have a larger mass of cold material. It would be ideal to "correct" for this problem by weighing the salt you will add, and then adding an equivalent weight of something else like salt-free sand to the other bucket. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
June 7, 2012
publicly reply to Ted Mooney
Q. What is the colour of pH paper of carrot juice?
Sonali Godansha- Balotra Rajasthan India
July 26, 2015
publicly reply to Sonali Godansha
![]() Hi Sonali. You forgot to attach your photo of the pH paper you dipped into carrot juice so that we could name the color for you ... or maybe I misunderstood the question?
Hi Sonali. You forgot to attach your photo of the pH paper you dipped into carrot juice so that we could name the color for you ... or maybe I misunderstood the question?
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
July 2015
publicly reply to Ted Mooney
A. Litmus paper is a terrible indicator as the range of color change is wide from either red to blue or blue to red.
A short range pH paper would be far better.
The range of carrot juice is any where from 4 to 7, depending on the carrots used, or if it is a commercial juice or the pH of any water that you might use to dilute it. Even how much air that you beat into it will have an effect.
A problem-- Carrot juice is a good stain or dye that will make the pH paper a bit difficult to read and terrible in the yellow/orange range.
- Navarre, Florida
July 26, 2015
publicly reply to James Watts
A. There is a published color chart of red cabbage juice color vs. pH 1 - 14. You will find it in Rhodiums Drug Chemistry Archive. Besides all the information on how to commit felony crimes (don't get the wrong idea, folks) there's also info on how to purify and dry solvents, properties of common acids/bases, etc.

Dave Wichern
Consultant - The Bronx, New York
October 26, 2015
publicly reply to Dave Wichern
![]() Thanks for the fascinating post, Dave. I found several archives of Rhodiums Drug Chemistry, and several cabbage juice pH charts, but couldn't tie the two together.
Thanks for the fascinating post, Dave. I found several archives of Rhodiums Drug Chemistry, and several cabbage juice pH charts, but couldn't tie the two together.
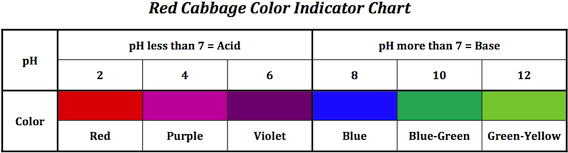
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
October 2015
publicly reply to Ted Mooney
A. It is under the "Equipment and Lab Technique" section, with the title "Natural pH indicators" There's also a chart for red beet juice, which is good in the pH 11 - 14 range.
There are also articles on how to build your own magnetic stirrer, your own fume hood, your own glove box, and glass blowing.
How I know about this is I used to sell lab chemicals in Berkeley, CA, and the owner put me in charge of determining whether people were buying chemicals to make dope, explosives, poisons, etc. The lighter side of all this was hearing addled burnouts try to pronounce "phosphorous." Sometimes, they'd put their girlfriends on the phone, 'cause girls can't be criminals. Ha, ha!

Dave Wichern
Consultant - The Bronx, New York
October 26, 2015
publicly reply to Dave Wichern
Q, A, or Comment on THIS thread -or- Start a NEW Thread
