
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Science Project: Coins in vinegar
Q. Hi,
I wanted to find out what happens to coins after they have been in vinegar ⇦in bulk on eBay or Amazon [affil links] (brown, white and red wine vinegar) for 1 week.
Thanks heaps,
Emma R and Serena B [last name deleted for privacy by Editor]- Sydney, NSW, AUS
2001
2001
A. Hopefully they will be inspected and have a report written about them.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
P.S.: After they have been in the vinegars for a week, inspect them and tell us what you see and we may be able to help. The purpose of your assignment is to practice laboratory and scientific methods, and the very first rule is to simply do the experiment and record the results. If you are told what the answer "is supposed to be" you are likely to deliberately or unconsciously jury-rig the experiment (give too much weight to agreeable results and explain away contrary results as errors) until it gets those desired results; that plague is called "junk science" -- and we've already seen too much of that from both sides on the global warming debate -- we certainly shouldn't teach it to grade school kids :-)
![]() My thoughts exactly, Ted....BRAVO!
My thoughts exactly, Ted....BRAVO!

Marc Green
anodizer - Boise, Idaho
2001
A. After coins have been dipped in vinegar for a week the penny will be cleaned of all the dirt on it and the rest of the coins will stay the same. I know because thats what I did for my science fair experiment. It's due tomorrow. Wish me luck !
Diamond H. [last name deleted for privacy by Editor]- Bronx, New York, USA
2004
 I am currently trying out coins in vinegar and I disagree with your other writer's reply. The coins corrode and grow mold, they will eventually leave you with only copper remains.
I am currently trying out coins in vinegar and I disagree with your other writer's reply. The coins corrode and grow mold, they will eventually leave you with only copper remains.
- Sydney, NSW, AUST
2004
 I disagree my coins changed tremendously, my quarter has lines going vertically on the coin and the rest of the coins has blue particles of something on them, the dime stayed the same.
I disagree my coins changed tremendously, my quarter has lines going vertically on the coin and the rest of the coins has blue particles of something on them, the dime stayed the same.
- Barnwell, South Carolina, America
2006
A. "Coins" are made of various metals, and the reaction you get will depend on what metal the coins in question are made of. For example, U.S. Pennies from before 1982 are solid copper and can react with vinegar much differently than pennies from after 1982 which are made of zinc with a copper plating (depending on how free of porosity and pinholes the copper plating is).

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2006
Q. I am a year 8 student undertaking a science experiment by place coins in vinegar and leaving them for 7 weeks to see what the effect will be on the coins.
I was wondering:
I have 7 coins from different countries around the world and they are made up of different metal components. Should the vinegar have a different effect of each coin or will vinegar have the same effect regardless of the metal components?
Also I have placed these coins in glass jars the lids and I am not removing the lids over the experiment. I have noticed that my coins are not showing the same signs as other people experiments that have no lids or the ones that are taking theirs out of the vinegar to examine. Why?
student - Thornton, NSW, Australia
2006
A. Mitchell,
Please ask your teacher if you can alter your experiment! Doing an experiment on the effect of vinegar on various coins when you don't even know what metals they are made of can't help you learn much.
But on the other hand you've made a great observation about the effect of lids. This sounds like a much more worthwhile experiment to pursue, and suggests that there is a strong untracked variable at work, corrupting the experiment the other kids are doing. Plus it will be far more interesting for you because it is based on an observation that you made yourself. Best of luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2006
Q. Hi, I'm in 10th grade and I need to figure out what the chemical equation is for the reaction of copper and vinegar. Could you please help me out. Thanks
Rachel
student - encinitas, California
2007
Rachel, give us the left side of the equation and I'll supply the balanced right side for you. But without the left side we don't know where to start, i.e., what you understand and what you don't. For example, whether Cu0 means anything to you or it doesn't. Thanks.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2007
Q. Is there anything that will stain a coin? I'm doing a lot of experiments on coins whether or not they can lose their color or change color at all. Give some more ideas for experiments too.
KC R. [last name deleted for privacy by Editor]- Mapleton, Utah, United States
January 31, 2008
Hi, KC --
You have to really think through exactly what you mean by "stain" before you can take the next step. If you have only a fuzzy concept of what you mean by your own words, you won't be able to prove or disprove them but will just run yourself in circles. For example: if you paint the coins, does that count as staining them to you? If they get slightly tarnished so they are not the bright shiny way they were when new, does that count as staining them? If you use sandpaper to partially remove the finish from a penny, does that count as staining it? Good luck, but you first challenge is precise wording :-)
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2008
March 25, 2008
Q. Hi!
I am doing a science fair experiment, and I would like if you can help me to answer some questions. The answers that you gave to the other people helped me to answer other questions.
- what is the effect of acids in metals?
(everywhere it says that is cleaning the coins, but I don't really know)
-where are the coins made?
(is it in a special factory with a special name, or just anywhere?)
- how do you recognize fake coins? what are they made of?
Wish you can help me.
My special thanks
school work - Colombia
A. Hi, Camila --
Acids can dissolve the tarnish on coins that has discolored them, and some people might call that "cleaning them" -- although that is not strictly correct as you will learn from our FAQ on "Cleaning" Pennies. If the acid is strong enough, and the coin left in it long enough, the acid can dissolve the coin.
The place where coins is made is usually called a "Mint". There is probably an official government-operated "Columbian Mint" but I don't know. There is a US mint, which you can read about at www.usmint.gov.
There is no worry about fake coins for low value coinage because the metal costs more than the face value. No counterfeiter is going to spend 10 cents of time and metal to make a 5 cent counterfeit coin :-)
For more valuable coins, one good indication is weight. Gold is heavier than lead, and much heavier than most common metals; so if a coin is not heavy, it's not gold. Good luck with your project!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2008
March 29, 2008
Q. Hi again, it's Camila!
Thanks again for your help, and I need some more
-I need to know which metals are used to make fake coins
I've tried in google to search about it but no one gives me an answer
-how do you recognize fake coins? apart from the weight?
and I forgot the last time to put my grade. I am in 7th grade
bye
Thanks
School Work, Student - Colombia
April , 2008
A. Hi, again, Camila. As I mentioned to KC, you really need to carefully think through and define what you mean by "fake coins" before you will make much progress. Do you mean something you can put into a gumball machine which judges a 5 cent piece only by it's size, or do you mean something sophisticated enough to fool a bank teller into thinking it's a real gold coin worth a lot of money?
The former you could probably cut out of wood. The latter would need to be a heavy metal (lead might be heavy enough if the teller isn't paying careful attention), and would have to be gold electroplated. After you think about what coin would be faked and for what purpose, I think you'll be able to answer your own questions. But there are both chemical tests and X-Ray tests that can tell people what metal a coin is made of. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Students please search the site for similar letters or read our F.A.Q. on Cleaning Pennies.
Q. Hi
I am about to undertake an experiment in my year 8 science class where the aim is to identify which coins are most affected by standing in vinegar, but before I begin, I have to decide on what my dependent variable is going to be, and I'm unsure what I should be measuring? Could you please help?
Thanks
- Alstonville, NSW, Australia
July 27, 2011
A. Hi, Jack.
You will be trying to determine which coins are most affected by standing in vinegar, so it sounds like the thing that you will be varying is which coin you put in the vinegar, and the thing you will be measuring is the effect the vinegar had on the coin. For grade 8, you probably don't have to go beyond simple visual indications of what the effects were. Is it corroded, pitted, did it turn black, did it lose it's shine, is it covered with rust, etc.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
July 27, 2011
Q. I'm doing a science fair project and I tried it but it didn't work. What did I do wrong?
I need some answers if ya'll be kind enough. I just don't understand what I'm doing wrong.
Jodi g [last name deleted for privacy by Editor]- dover-foxcroft, Maine
February 11, 2013
What you did wrong was you wore a blue dress rather than a red one while you did the experiment, Jodi.
If you don't know what I'm talking about, it's because you still didn't read our "F.A.Q. on Cleaning Pennies" yet as we've already suggested twice previously :-)
Luck & Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
February 12, 2013
Q. Hi, my son is in 4th grade and wanted to see which liquids cause coins to rust. He only tested quarters and pennies-- all from years 2009-2013. He has since learned that since coins do not contain iron, they will not rust, but rather will oxidize. He has completed his project but is working on the analysis part. vinegar changed the color of the quarter (I assume this is copper oxide), soda changed the penny (zinc oxide?), and there were slight changes from the saltwater and lemon juice. Now, our question is why? I know that vinegar and lemon juice are acids but there are many discussions online about how acids will clean the coins--why did they corrode them or oxidize them? Is it possible that because the coins soaked in the liquids for 2 weeks, that the outer coating corroded and the inner material is what is oxidizing?
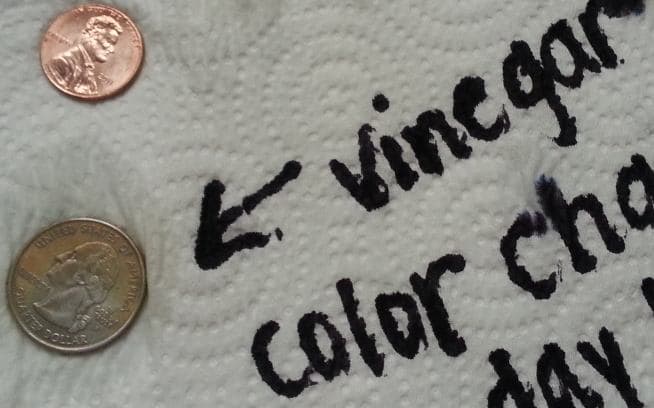

I wanted you to see how the vinegar had changed to bright green also. Interesting. Is this a copper solution of some type?
Thank you for any information you can provide. He has read a book on metals and have checked several websites concerning the topic.
Wendy K [last name deleted for privacy by Editor]mother and student - Greensboro North Carolina USA
November 25, 2013
A. Hi Wendy. If you look at the edge of a quarter you will see copper; the coin is "clad" with skins of a copper-nickel alloy.
Based on your observations (copper colored tint on the face of the quarter, plus the greenish color to the vinegar), what almost surely happened is a "displacement plating" / "immersion plating" reaction of nickel dissolving into the solution (dissolved nickel is green), and copper from the edge plating out onto it.
This is not really different (except in speed & degree) from another experiment kids do where an iron nail is put into blue copper sulphate solution and the copper plates itself out onto the nail.
The vinegar was apparently unable to attack the copper coating on the penny (which is not surprising, it's not supposed to). If the copper coating on the penny were compromised and broken or porous, the vinegar would have attacked the underlying zinc core.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
November 2013
August 19, 2015
![]() I happened to Google "Vinegar dissolves coins " and this thread popped up.
I happened to Google "Vinegar dissolves coins " and this thread popped up.
I thought it kind of funny, I had a fruit fly problem, so I turned to Google and found they are insanely attracted to apple cider vinegar, so I got an old mason jar and filled it with the vinegar and it worked, but not as well I had I hoped.
I added a few coins to the jar some silver and copper(all US Coins, various coins and ages) and just let it be. I'd move it around the kitchen when I cleaned, but figured it's gone on this long, why not let it go?
So I happened to look at it once again and sure enough, all I have left are two pennies and the vinegar has changed colors. Quite an interesting little experiment.
My only suggestion to prospective students wishing to repeat would be start this at the beginning of the school year and take pics twice a week to document the process. Take notes documenting changes.
Part of me wants to hang an old junk necklace and see if the dissolved metals will coat the chain in some fashion. Although I imagine it needs some sort of current to do so. Kids don't try that at home.
Glad I stumbled upon your website and was able to share my kitchen counter experiment.
- North of the Old Dominion
DOES vinegar RUST OR NOT!!
Q. I am a girl in grade 5. For our major science experiment, we were rusting nails in different substances. Nothing rusted better than the vinegar. For science home work, I had to find out why vinegar rusts (that's not my question). Some sites said:
- vinegar rusts
- vinegar removes rust.
- vinegar doesn't rust.
Which, if any of them, are right!
- Perth, WA, Australia
September 12, 2018
September 2018
A. Hi Sammy. Regarding the first assertion, the obvious answer is that under some conditions vinegar causes nails to rust. You know that because you did the experiment and proved it.
Regarding the second assertion, the way to prove or disprove it is to dip a slightly rusted nail into vinegar and see if at some point, maybe after only an hour or two, the nail has less rust. I suspect that it will.
You know the third assertion is false from your experiment. But it may need clarification or specific parameters. One of your classmates could have dipped a nail into vinegar for 2 seconds, then rinsed and dried it, and correctly claimed that it didn't rust.
Take a lesson from Richard Feynman, one of the world's most accomplished nuclear physicists and best teachers :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread
