
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Acid Resistant Coating for Aluminum
September 8, 2021
Q. I have a part that is made from bar stock aluminum grade 6061-T6 or 5052-H32. Its finishing specs are as follows:
1. NICKEL STRIKE OVER ZINCATE IMMERSION
2. ELECTROLESS NICKEL PER ASTM B733 (0.0003"-0.0005")
3. NICKEL STRIKE
4. CHROME PLATE PER ASTM B456 (0.000010" AVG MINIMUM)
The part is in direct contact with 10-15% concentration of sulfuric acid liquid and vapors heated.
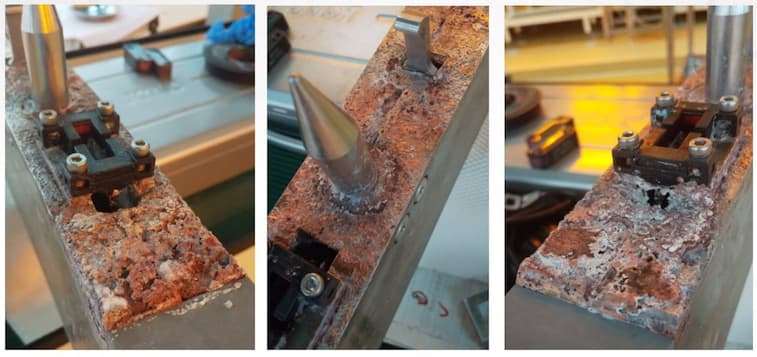
The issue is the life expectancy is less than 2 years before it corrodes. I was asked by my boss to look at alternative coatings and different grades of aluminum. My question is are there alternative aluminum coatings some one can recommend?
Frank DeFilippi- Billerica, Massachusetts
September 2021
A. Hi Frank. Any place there is no wear, organic coatings like vinyl plastisol would be better, but it looks like you are dealing with some sort of centering post where a metallic coating is required.
I can't imagine chrome contributing much to the corrosion resistance, and assume it's there for lubricity.
You might try teflon impregnated electroless nickel as an alternative. The plating could be thicker, say 0.001", but my bigger question is why make this out of aluminum?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
⇩ Related postings, oldest first ⇩
Q. Is there a coating for aluminum to make it HCl resistant ? I am running a process that has HCl in it and I am proposing to use an Aluminum chamber to do this. Therefore I need to protect the aluminum from the HCl, so it will last for 5-10 term.
Hugh Walton- Auburn Hills, MI, USA
2001
A. 5-10 what - days-weeks-months- years.
What concentration and what temperature and what use- a few minutes a day or all of the time?
On the low end, 2 thousandths of high Phosphorous electroless nickel will work. For long term, use an appropriate specialty stainless steel.
For low budget, can you get a blow molded thick polyethylene container that you might possibly "hide" in a metal cylinder?
James Watts- Navarre, Florida
2001
A. It is possible to design in a glass reactor? Aluminum is amphoteric, meaning it will react with acids and bases, and probably not a good choice with HCl. If you really need the reactor to be metal, you may have to go with tantalum, titanium, or maybe even gold or platinum. Some stainless steels and grades are better than others at resisting chlorine attack, but none are particularly suitable for HCl.
Dale WoikaSurface Conversion Sciences - Bellefonte, Pennsylvania, USA
2001
A. Try using a fluoropolymer such as Halar ECTFE, we coat a lot of parts that use acid baths and this type of coating gets the nod. Usually around 15-30 mils thick.
Josh SonjuSonju Industrial Inc. - Kalispell, MT, USA
2001
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. I am looking for an acid resistant coating for machined aluminum machine parts.These parts need to have a similar resistance as 304 stainless.
Brad Hartley- Media, Pennsylvania
2001
A. The simplest answer to this is to pre-treat the aluminum parts with chromating process and apply Teflon coating or polyester powder coating. If the parts require to withstand hard mechanical scratch, then hard anodising with hot seal may help your application.
Andy Lim- Kuala Lumpur, Malaysia
2001
A. If you use hard anodizing process because you want a scratch resistance coating. It is much better off to leave it un-sealed. The sealing may reduce the scratch resistance of the surface.
S. Y. Yuen- Hong Kong, China
2001
2001
A. Sir:
Have you tried preparing the Aluminum by etch/desmut/zincate/Electroless Nickel Strike/Electroless Nickel(high phosphorus)? The high phosphorus deposit will give you excellent corrosion protection, plus have coverage wherever the solution makes contact with the Aluminum

Ed Budman [dec]
- Pennsylvania
With deep sadness we advise that our good friend Ed passed away Nov. 24, 2018
Q, A, or Comment on THIS thread -or- Start a NEW Thread