
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Cleaning Porous Pot Cathodes - There's gotta be a better way!
Q. Hello all!
I'm new to the world of Chrome plating- so new, in fact, that I've just had to clean my first cathode! It reminded me of how much I miss anodizing. It's so much less obnoxiously filthy.
The current [see what I did there?] procedure is to haul it out by crane into a cut-off 55 gallon plastic drum, pull the cathode, put on ALLLL the PPE, and sit there sweating with a paint scraper for an hour or two...
I cannot believe this is the only way to clean these things. It's not even all that effective!
There has to be a better way. Acid soak? What acid? Electricity or nah? Reverse polarity in a dedicated tank to drive off the scale?
For reference these are carbon-lead cathodes. If anyone has a trick they would like to share, I'm all ears and will be very grateful. Bonus if your shop has a silly name for them... Time to clean the Lobstah Pots!
Thanks in advance!

Rachel Mackintosh
- Greenfield, Vermont
September 13, 2024
for Shops, Specifiers, & Engineers

by Weiner & Walmsley (1980)
avail from eBay, AbeBooks, or Amazon

by Robert K. Guffie (1986)
avail from AbeBooks, or Amazon

avail from eBay, AbeBooks, or Amazon
"Hard chromium plating: A Handbook of Modern Practice"
by John David Greenwood (1971)
avail from eBay

very rarely avail from Amazon
but copies are available in a few libraries)
"A Chromium Plating Bath With The Fluoride Ion"
by Alfred Perlenfein (2013)
avail from eBay, AbeBooks
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
A. Hi Rachel. I'm sorry that no one answered you after all the help you've offered others. I can only offer book knowledge ...
Guffie does not mention porous pots but talks about cleaning tank anodes on p.69-70. Paraphrasing: if you have a lot of yellow lead chromate it's because the anode current density is too low. He says the only time they should need cleaning is if they've been unused for 3 or 4 days and that there are proprietary solutions which the anodes can be simply dipped in, but if the buildup is so severe that chemical cleaning won't do, use only a tampico brush.
Weiner & Walmsley do not mention porous pots either. But on p.180, they say take the anodes out when not in use if practical and rinse and dry them. They too say that lead chromate won't build up when in use, only when idle. They say you can loosen the coating by a simple soak in 10% caustic and 10% rochelle salts.
In Clarence Peger'd book, p.77, hr mentions your scraper method for tank anodes. He discusses porous pots a little bit on p.250, but not how to clean their cathodes.
However, elsewhere, Peger offers rather complete operation & cleaning instructions on-line. In that article he says leave the assembly in the tank, undo the two screws and remove only the cathode for cleaning, pump out whatever is in the pot, then spray rinse the inside of it. However, you may be using a different brand or style of porous pot.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. Hello, there!
I don't know if I understood Rachel's question, I'm not sure I know what a "Porous Pot Cathode" is.
But from Ted's answer, it looks like you wanna clean the chromate film from lead anodes that are used in chrome plating.
If so, here we clean our anodes once a month. We remove them from the solution before the weekend and put them in a solution composed of 10% caustic soda and 4% rochelle salt until Monday.
On monday morning, before work, we brush the anodes with a wire brush, wash them and start plating. It's still hard work, but probably easier than raw cleaning them :)
Hope it helped.
Chemical Engineer - Nova Friburgo, Rio de Janeiro, Brazil
January 21, 2025
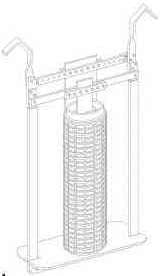
(Hard Chrome Plating Consultants, Cleveland)
A. Thank you Pedro.
A porous pot is a device used to remove contaminants from chrome plating baths and re-oxidize trivalent chromium to hexavalent. It is an assembly consisting of a lead cathode hanging into an unsealed (porous) ceramic vessel with a lead anode in close proximity on the outside of the ceramic vessel. Usually the porous pot assembly sits inside the plating tank. The contents of the ceramic vessel start out as chrome plating solution, but due to the electricity flow, the contents are soon rich in iron and other cationic contaminants which are dumped to a container for waste treatment.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread
