
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Hard Chrome VI plating throwing power problem
Q. Hi,
I have included a hull cell test panel picture for everyone's inspection.


Our issue is with small components on a jig, that display the usual dog bone when plated. We are trying to even out the plating so that the area near the jigging point and the edges all plate within spec. the inner part is in spec and the out above, or the inner is below spec and the outer in spec, for thickness.
I am running a HEEF25 solution at 267 g/l chromic acid, 3.0 g/l sulphate, and HEEF25 catalyst added as per the data sheet, ~300 mls per 1000 amp/hrs.
Would the addition of a robber wire around the outside of the part be of help?
I would be grateful for any help or suggestions.
Thanks and best regards,
Mark
- A rainy rock in the Irish sea
September 6, 2024
by Weiner & Walmsley

on AbeBooks
(rarely)
or eBay
(sometimes)
or Amazon
(sometimes)
(affil links)
by Clarence H. Peger
(You're unlikely to find this for sale ... but copies are in select libraries)

from AbeBooks
* rarely available *
or eBay
* rarely available *
or
Amazon
* rarely available *
(affil links)
by Robert K. Guffie

on AbeBooks
(rarely)
or eBay
(rarely)
or Amazon
(affil links)
A. Hi Mark.
I am not a hands-on chrome plater, so the information I can offer is a bit vague, but there are three ways that I can think of to further control the current distribution after the racking arrangement and anode arrangement has been settled: auxiliary anodes, shields, and robbers.
• 'Auxiliary anodes' are most useful for plating in recesses and I.D.s, but they don't sound applicable for your situation.
• 'Shields' are pieces of non-conductive plastic placed in between the anodes and the pieces you are trying to plate, their purpose being to force the electricity to 'take the long way around' and therefore not build up as much in high current density areas. They are used in lots of plating applications but only rarely in chrome plating because chrome plating is so inefficient that it's hard to make a shield that just reduces the plating thickness rather than stopping it dead. I have personally not heard of shields being used in hard chromium plating, but Weiner & Walmsley apparently have ⇨
• 'Robbers' or 'thieves' are indeed scrap pieces of wire attached to the cathodic side of the circuit with the aim of stealing some of the plating from the high current density areas. They are quite common in hard chrome plating. The robbers don't actually shield the electrical path to the parts; rather, they are very slightly above and below their ends to draw current away from the ends.
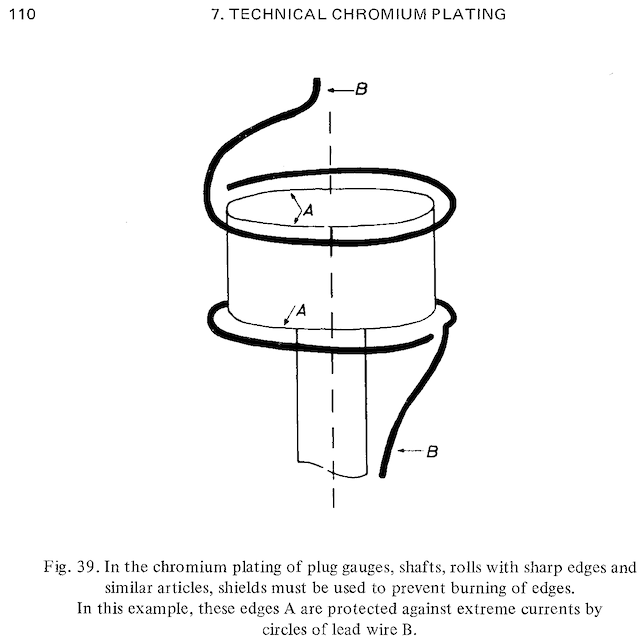
(from Weiner & Walmsley)
Weiner & Walmsley has a pretty good subchapter with illustrations of the three approaches, but in a rather abstract way rather than carefully detailed.
Clarence Peger's "Chrome Plating Simplified" is maddeningly detailed but unavailable except for this short extract, but someone you know just might have a copy. Peger believed exclusively in the "reversible rack 2 bus bar system" where carefully designed conforming anodes, carried around with the rack as one combined fixture, insure even plating thickness.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
|
|
- A sunny rock in the Irish sea September 19, 2024 A. Hard chrome: Electroplate consultant - Sterling Heights September 22, 2024 |
Q, A, or Comment on THIS thread -or- Start a NEW Thread
