
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Dissolution of silver anode in high cyanide solution
Q. Hi. I have immersed a basket of silver anodes (>99% purity) into silver plating electrolyte with around 150 g/l of KCN for several days (rectifier is off). Would it dissolve the silver from anode into solution? Thanks.
Cody Foong- Malaysia Penang
December 8, 2020
A. Hi Cody. Metallic silver is soluble in potassium cyanide so, yes, to some degree the silver will dissolve --
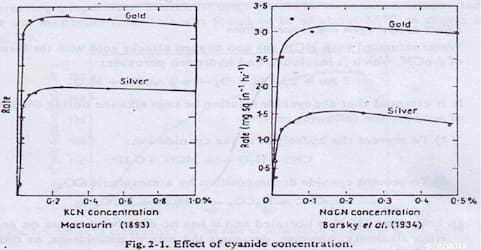
(from 911metallurgist.com/blog/gold-silver-dissolution-cyanide-concentration)
But please, no hypothetical questions ... you told us what you actually did, so what was the actual result? And what motivates your question? Thanks.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
December 2020
Q, A, or Comment on THIS thread -or- Start a NEW Thread