
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

Thread 595/71
Corrosion failure on zinc plated rod
March 26, 2014
Q. We are an automotive component supplier with a concern on a 4 mm diameter zinc plated rod. This is part of a dynamic assembly. The rod is corroded with an orangish rust looking appearance. The corrosion is only at the contact surface that mates with a felt washer that is approximately 10 mm in length and 10 mm in diameter. this assembly goes into a 10 mm ID galvanized tube. The tubes are exposed to a cleaning solution with the following MSDS elements
- 80-90% Water, CAS #7732-18-5; TLV/TWA (ACGIH): n.e.; PEL/TWA (OSHA): n.e.; Oral: n.e.; Dermal: n.e.; Inhalation: n.e.; Skin Effects: n.e.; Eye Effects: n.e.;
- 3-5% Sodium Metasilicate, CAS #6834-92-0; TLV (ACGIH): n.e.; TWA, PEL (OSHA): n.e.; Particulate not otherwise classified: TLV (ACGIH) - 10 mg/M3 (total dust); TWA, PEL (OSHA): 15 mg/M3 (total dust) 5 mg/M3 (respirable dust); Oral LD50 (rats): 847 mg/kg (Rhone Poulenc Chemicals), 1153 mg/kg (RTEC 7/91); Dermal LD50 (rabbits): 1153 mg/kg; Inhalation LC50 (rats): n.e.; Skin Effects (rabbits): Corrosive (4 Hr. exposure, moistened skin), Non-irritant (4 Hr., dry skin) (Rhone Poulenc Chemicals); Eye Effects (rabbits): Corrosive (Rhone Poulenc Chemicals);
- 1-10% Sodium Xylene Sulfonate, CAS #1300-72-7; TLV/TWA (ACGIH): n.e.; PEL/TWA (OSHA): n.e.; Oral: n.e.; Dermal: n.e.; Inhalation: n.e.; Skin Effects: n.e.; Eye Effects: n.e.;
- 1-10% Alkyllaryl polyether alcohol, CAS# 68412-54-4; TLV/TWA (ACGIH) n.e. ; PEL/TWA (OSHA) n.e.; Oral LD50 (rats): >5000 mg/kg,(Rhone & Haas); Dermal LD50: n.e.; Inhalation LC50: n.e. ; Skin Effects: n.e.; Eye Effects:
The felt appears to have acted as a sponge and absorbed some solution, then after sitting for some days/weeks the rod corrodes. This is causing a concern at the customer. Which chemicals/solutions may be contributing?
automotive component manufacturer - aberdeen, North Carolina, usa
publicly reply to brad roback
A. Hi Brad. Water, for one.
A reasonably thin zinc plating is not going to resist this wet condition for long at all. I think this rod needs to be electroless nickel plated, or made of stainless steel, although heavy galvanizing might be enough for a short life. Unfortunately, even the galvanizing on the inside of the tube may not last long.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2014
publicly reply to Ted Mooney
March 31, 2014
Q. Thank You Ted:
I am following up on this for our company. We had the parts chemically analyzed and the results indicated the part was corroded by Aqueous Chloride.
The report indicated the Chloride was not coming from the cleaning agent used, however, the cleaning agent is diluted with ordinary tap water and we assume this is the source of the Chloride. We are having that analyzed.
What thickness of Nickel is recommended for good corrosion resistance?
Obviously making sure the product is dry when assembled is key but would deionized water slow the corrosion process at all? This question relates to possibilities for consuming our current rod inventory while new coatings and materials are investigated.
Automotive Component Manufacturer - Aberdeen, North Carolina, USA
publicly reply to Randy Miller
A. Hi again. Yes, I believe that if the cleaning solution is made up with de-ionized water you would be somewhat better off than with tap water. Unfortunately, I don't understand the situation. When you says it's exposed to a cleaning solution, do you mean it's exposed once before assembly, such that if properly dried it stays dry for the rest of its life? Or do you mean it's periodically exposed to this cleaning solution? Because simply, zinc plating will not stand up to frequent/constant wetness.
If it's going to be wet, the rod and tube should be made of stainless and, if that's prohibitive they should probably be hot dip galvanized. Electroless nickel would need to be thick enough to be pore-free, which, depending on the smoothness of the rod, might require as much as .001" thickness. But if one part is electroless nickel and the other is hot dip galvanized zinc, you have a possibility of galvanic corrosion if wet and if there is metallic contact (through any metal path) between the rod and the tube.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2014
publicly reply to Ted Mooney
April 1, 2014
Q. Thanks Ted:
Sorry for the confusion. At this point we are still root causing. All we know is that at some point the interior of the assembly became wet with something that contained chloride.
The assembly is comprised of a galvanized tube that houses a spring wrapped around a steel rod (zinc plated) the spring can contact the rod but the rod cannot contact the tube and the spring is masked off from contacting the tube. It is possible for the spring to contact the tube at each end were the spring clips into a hole in the rod so this area could provide a metal to metal path of contact (rod to spring to tube).
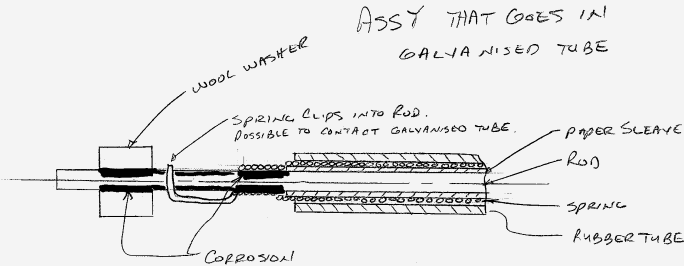
The corrosion is happening in one general area, under a wool washer that is placed on the rod to keep the rod centered in the tube and where the spring OD necks down and is in direct contact with the rod. This area is also were the spring clips into the rod (the metal to metal path of contact).
The only moisture introduced during our manufacturing process is a cleaning operation of the tube prior to assembly. The tubes are cleaned with the diluted cleaning agent, rinsed, and then inclined until dry.
We are assuming at this point that the moisture came from wet tubes being taken to the assembly line because after assembly it would almost need to be immersed to get wet inside the tube and we feel we would see indications of water damage on the rest of the assembly.
Automotive Component Manufacturer - Aberdeen North Carolina, USA
publicly reply to Randy Miller
A. Okay, I had in fact misunderstood, and had thought that getting wet with a cleaning solution was a planned operation. If the rod is properly rinsed (DI water is preferable but probably not mandatory), and the tube and rod are dried properly before assembly, I wouldn't expect corrosion problems. I believe wet assembly was the issue.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2014
publicly reply to Ted Mooney
April 4, 2014
Q. In further research we have discovered that our felt washer contains up to 2.5% of Methyl Chloroform and when combined with water the result is Hypochlorous Acid. Some research indicates this is corrosive to metals. Do you feel this could be a contributor? We went down this path in our research because the corrosion is always under or near the felt washer.
In our containment efforts we decided to tear down a sampling of product that has no noise to verify there is no corrosion. What we found that some parts that make no noise have signs of some corrosion. On these parts the Zinc has started to pit and in some cases small flakes of Zinc have come off the rod.
The question is, if these parts never get wet again will they still continue to corrode?
Automotive Component Manufacturer - Aberdeen North Carolina, USA
publicly reply to Randy Miller
A. Hi Randy. Any acid and any chloride is corrosive to zinc plating, of course, but I still feel that the principal problem is just the moisture, because zinc will corrode if packed wet even when well rinsed and no "corrosives" are present. If zinc has flaked off, the parts are no longer properly zinc plated. Personally, I don't think the assemblies are salvageable. Proper rinsing and drying is imperative in all cases, but when there is a sponge-like device that will hold the moisture there, it's even more so. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
April 6, 2014
publicly reply to Ted Mooney
Q, A, or Comment on THIS thread -or- Start a NEW Thread