
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Patina sprayed on copper doesn't look good
Current question and answers:
March 2, 2021Q. Please help:
I know that this looks a lot like the kind of copper patina possible with heat or liver of sulfur but It is NOT.

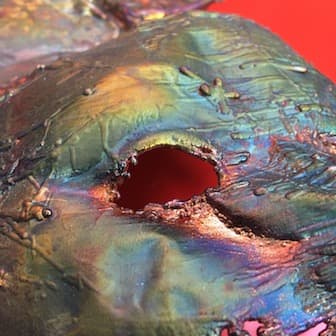


I'm getting this effect using only tap water and baking soda. Is it possible to explain what is happening? It's irregular and hard to control, and I'm looking for help. It is possible some heat (hot water seems more effective) but honestly I can't reproduce it on demand. Sometimes its very colorful, sometimes only in spots- and sometimes not at all.
Note - I'm using a basic 1900's copper acid formulation using Thiourea
⇦ this on
eBay
or
Amazon [affil links] . It is a bright finish.
Any help or pointing to resources would be appreciated. Thanks! and stay safe
best
Raphael
- Brooklyn New York
A. Hi Raphael. I have seen this general look countless times: it is interference coloring. The colors are not reaction products or pigments, but result from some of light bouncing off the outside of the object and some of it penetrating a very thin (partial wavelength) transparent or translucent coating on the object and bouncing off that. The reflected light from the too halves interferes and causes this. Similar effects are seen on carnival glass, titanium anodizing, and a drop of oil on a mud puddle, and even the new trend of "oil slick hair coloring".
I think you may find companies now selling chemicals that will do this. Exactly why it's happening to you somewhat randomly, I don't know ... perhaps the baking soda is reacting with brighteners from the copper electroforming.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2021
March 3, 2021
Q. Thanks Ted! Though to be clear this is not the result of patina spray (which seems to be the category I've been appended to).
This has something to do with rinsing the electroformed object after removing it from the plating bath.
I've done some research online for commercial products that create this interference effect and they ALL seem to be some variety of liver of sulfur. Is it possible that I'm making a dilute liver of sulfur formula with just the baking soda and Thiourea
⇦ this on
eBay
or
Amazon [affil links] ? Not sure of the chemistry there.
I guess what I'm wondering is if anyone can explain the chemistry behind this so that I can better control the process?
Thanks!
best
Raphael
- Brooklyn New York
A. Hi Raphael. Your theory apparently is that the sulfur in Thiourea ⇦ this on eBay or Amazon [affil links] is producing a liver of sulfur-like effect. I offered another possibility that addition agents are reacting with baking soda to produce a very thin oil-like coating which is causing an interference effect. The thread remains open for readers to offer their alternate theories of what may be causing this interference coloring. If you look up the SDS / MSDS for the chromatic hair colorings, I don't think you'll find liver of sulfur though.
I do try to append inquiries to the most applicable of our 60,000 threads whenever possible because google and other search engines offer zero coverage to new unanswered questions, which means the only readers who could ever see your question are those who start at finishing.com/letters during the period while your inquiry is on the current topics list, which is only a relatively small percentage of the total readership. Good luck in solving this because it's a beautiful look on your pieces!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
March 2021
A. DIY liver of sulfur can be be made by mixing melted sulfur and potassium carbonate...If you want predictable results you must use strictly defined working cycle (quality of rinsing water, its temperature, baking soda solution must be strictly defined, how long you immerse your objects in solution, etc.) Even then results can be quite different every time (electroformed copper quality is not same every time).
Hope it helps and good luck!
- Zagreb Croatia
March 5, 2021
⇩ Related postings, oldest first ⇩
October 17, 2012
Q. Hello All,
My name is Rob and I am attaching pictures of my copper gutters and leaders that were sprayed with a "patina solution" 4 months ago, that I bought from California. The gutters were hanged 1 year ago, taken down and cleaned with #0000 steel wool
⇦ this on
eBay or
Amazon [affil links] to regain the shine. My question is multi-faceted.





1. Will the copper continue to patina past what is seen in the pictures, or is this the end product?
2. Why does the copper turn brown when it gets wet and stays blue-green while dry?
3. Will the striped/zebra look go away?
4. Are my gutters ruined?...the gentlemen that did the work told me that this "starts the process" and will take time to completely turn patina green but I need real solid advice. Thanks for your time folks.
Rob
homeowner - Jackson, New Jersey
A. Hi Rob.
I'll give my opinion and hope that others will offer you theirs so there is a good balance. Let me apologize for the fact that, although this is a very serious matter to you, to me it is just an interesting technical issue to bloviate about, nor do I know that much about it.
Before addressing your specific questions, I'll tell you what I believe the overall problem to be: Beautiful patina work is the product of fastidious custom work by artists; contractors aren't artists and we can't expect their washing-down of gutters with patina solution to equate to what a bronze sculptor would do for his proudest public displays. That said, this does look bad and if I were the contractor, I think I'd give it a second try, lightly spritzing all the surfaces to minimize running.
1. The patina process will continue, but over a time scale of years and decades, not of weeks.
2. I can't exactly answer, but on a recent visit to Cape May, I was struck by the fact that the brown vs. green areas of copper canopies seemed to reflect how much water hit them. The brown color is more stable and the green more prone to rubbing/washing away -- which corresponds with pennies in circulation staying brown, while pennies found in the cracks of a boardwalk are green.

3. Over the years the patina will even out but I doubt that it will happen any time soon.
4. I don't think the gutters are damaged at all.
Additional discussions and pictures of copper patinas are on topic 5720 and 17, and searching the site for 'copper patina' yields dozens of threads. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Dear Mr. Mooney,
Your response is very very much appreciated. So, if I wanted to start over, how do I remove the existing patina chemical if it is at all removable? Thanks again.
- Jackson, New Jersey
October 18, 2012
Q. I have bright new copper gutters and roofs. What can I spray to turn it to dark bronze but not black. I tried that Liver of sulfur(?) but it turned it black and also it needed to be wiped on instead of spraying it. Any ideas?
Noel Sugue- Knoxville, TN
November 13, 2012
A. Hi Noel.
I must make the same comment about brown gutters as about green ones: while a skilled artist may be able to produce an artificial patina that is beautiful and natural looking, I don't think a contractor will achieve it with a quick spray of anything. But I think a purchased brown patina (that is, where the copper salt is already dissolved in the purchased solution) offers better prospects than trying to find an acid/chemical that will react consistently with the copper in your gutters. Best of luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
November 13, 2012
Q. I sprayed my leaders and gutters with a patina solution and even though I used a fine mist it it would run and leave Drip marks. How do you prevent that from happening? I tried a sponge, a paintbrush, a rag -- they all leave drip marks and it looks terrible. Thank you.
Valarie Kennedy- Shirley New York USA
August 8, 2019
August 2019
A. Hello cousin . I can offer you three answers but you're not gonna like any of them :-(
The first is that the way the manufacturers of premium brass chandeliers get a perfectly even drip-free lacquer finish is by applying six or seven very thin coats. And if that weren't bad enough, brass lacquer has solvents which evaporate faster than the water in patina solutions, so their approach of multiple dilute coats would be very time consuming for you.
The second way, which is way beyond my level, is to have artistic talent specifically at this. If you patiently search the site for "copper patina", you'll find examples showing that such runs are not necessarily bad if you have a real feel for them, and can see the forest for the trees and the trees for the forest, as you're doing it.
The third, and even more impractical way is to wait many decades. Asbury Park's fabulous "Carousel Building", Hoboken's gorgeous "Lackawanna Railroad station", and the renowned roofs of Bancroft Hall at The Naval Academy are examples of this third approach.



Hopefully a reader will give us a fourth approach which offers a degree of practicality :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. I tried many light coats of misting, still sometime drips and I wipe it off with a scrubby and try again. The downspouts are the biggest problem. I can get the gutters to look pretty good without drips BUT the leaders are impossible. I wanted to do my entire house but not getting too far. I have sprayed many items with no drips and it looks great but I'm stumped on these downspouts. I'm going to try one more time by wetting a rag and dabbing it on. Oh well I'll let you know, thanks for trying to help me, Valarie
Valarie Kennedy [returning]- Shirley New York USA
August 9, 2019
Q, A, or Comment on THIS thread -or- Start a NEW Thread