
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Solutions to Problems in tin-silver plating on copper seed layer
August 28, 2012
Q.
Hi, I currently face a concern with my Tin-Silver plating process and more especially the Copper seed layer removal following the plating and Photo-resist Stripping.
We demonstrated with some colleagues that in some case in my final Tin Solder, it could incorporate some Stannous sulphate. We assume that it is the result of the reaction of CuSO4/H2SO4 Copper plating bath prior to Tin deposition because of bad rinsing between both steps (Tin-Silver plating solution is MSA Based).
Even if the treatment between Copper and Tin plating is clearly identified as a weakness, we notice the following result:
If we use diluted Sulfuric acid/Hydrogen peroxide mixture to remove the Copper seed layer, we observe some delamination material from the Tin/Silver solder. If the material is not completely removed (It looks like a defect or particle embedded in the solder..!), we could characterize it and found Sn, O and S (I assume it is Stannous sulphate.. reaction between CuSO4/H2SO4 and Sn contained in MSA).
If I do the same experiment without the Hydrogen Peroxide, nothing happens. Solder remains without any delamination. As soon as I add Hydrogen Peroxide, Solder delamination could be observed. Oxidizing agent seems to have a critical effect.
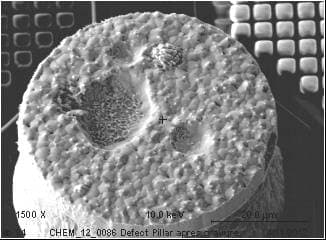
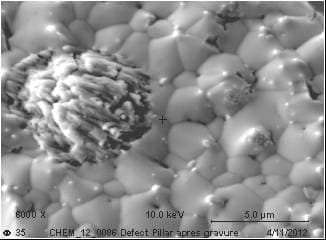
I tried some other chemicals than Sulfuric acid like phosphoric acid and nothing happens. It looks like only the Sulfuric acid + an oxidizing agent have a critical effect on what I suppose to be Stannous sulphate incorporated in the bulk Solder!
We focus on the Rinsing and any treatment prior to starting Tin plating but any Help or comment to understand the delamination reaction when using Sulfuric acid and Hydrogen peroxide would be really appreciate.
Thanks,
Have a good day
Mike
- Grenoble, FRANCE
Q, A, or Comment on THIS thread -or- Start a NEW Thread