
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Nickel surface treatment solution wanted
February 24, 2012
Q. Hi
I have a problem where nickel plating surface is not so good. As I think, the nickel process works well with Reel to Reel type plating.
I found nickel plating surface failure in the middle of working, there is no [known] changed working condition..suddenly happened.
Nickel plating surface got so many rounded projections (shown in photo / SEM x 3000).
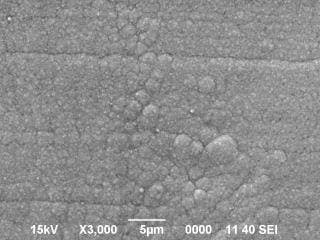
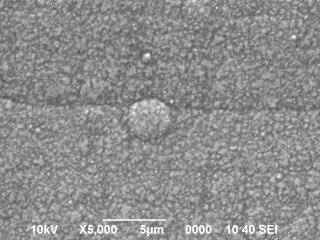
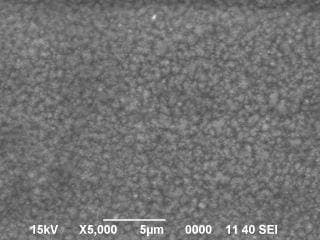
I don't know how to deal with it. I am waiting for your help!
-working condition-
1. Reel to Reel type / velocity : 4m/min / phosphor bronze
2. Nickel 600 g/l, boric acid 40g/l, Nickel Chloride 6 g/l
3. Solution temp. 55~60 degree
4. Current density 29(A) / voltage 5.5(V)
- Ansan city, Kyounkido, South Korea
Just to clarify: your nickel concentration is 600 g/L? Is that even possible?
- Brisbane, Queensland, Australia
February 27, 2012
February 28, 2012
I checked the concentration of nickel.
600g/L is right..
Nickel bath volume is 300 Liter. Net nickel solution volume is 250 Liter.
- Ansan city, Kyounkido, South Korea
February , 2012
Hello, Hyo.
You undoubtedly mean 600 g/l of nickel sufamate, Ni(NH2SO3)2 or Ni(NH2SO3)2.4H20, not nickel. 600 g/l nickel would indeed be impossible.
This looks like the beginning of nodule growth, which in turn sounds like brightener/addition agent problems, although we probably can't rule out a mechanical failure of a solution jet or something like that. Can you do a hull cell test or a stress tabs test to see if anything can be learned about what happens at other current densities?
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread