
-----
How can electroplating cathode current have efficiency less than or more than 100%?
Q. Dear Prof,
I am producing copper ternary alloy via electroplating, and work on the parameter that affected the produced alloy. My question is - Is any relation between cathode current efficiency and deposition rate? Could you please elaborate more on this? I really appreciate your attention and kind assistance.
Thank you in advance Prof.
- Johor Malaysia
May 16, 2021
May 2021
A. Hi Ita. Yes, there certainly is a relationship between current efficiency and deposition rate, but I'm not sure quite what your actual situation/question is --
• As a general rule the efficiency will drop as the deposition rate increases.
• As a general rule the deposition rate will increase as the efficiency increases.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 19, 2021
Q. Thank you very much Prof for the answer.
Could you please check on my formula, is it correct? If it is wrong, could you please give me the correct answer?
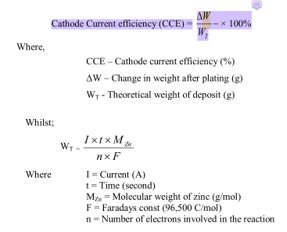
I am sorry for the very basic question. Thank you again for your kind help.
Ita Rosley [returning]- Johor, Malaysia
A. Hi again Ita.
• Your first formula is correct but obvious because it's the definition more than a formula :-)
• Your second formula is correct, but as a matter of style I would use A-sec/mole rather than C/mole as the units for the Faraday constant because your formula uses Amps and seconds, so switching to Coulombs just muddies the water.
• Your third formula is correct, just a bit hard to follow because time is expressed in seconds in your second formula but in hours in your third.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 2021
![]() Thank you very much, Prof. I really appreciate your help.
Thank you very much, Prof. I really appreciate your help.
- Johor, Malaysia
May 22, 2021
⇩ Related postings, oldest first ⇩
Q. Hello,
I am a materials engineer student and part of my degree entails the basics of electroplating, or electrochemical technology.
I wondered whether you were able to answer my question, as well as give some further, more in depth reasoning behind it as to further help understanding of the subject for my summer exams:
"In electroplating, how can a cathode current mechanistically have a current efficiency less then 100% and in some cases more than 100%?"
Thanks so much for your help.
Materials Student - Crowborough England
April 12, 2011
A. Hi, Alastair.
Let's say you have some Nickel Chloride (NiCl2) and HCl dissolved in an aqueous solution, and you put two nickel electrodes into that solution and connect a battery between those two electrodes, making one of the electrodes the cathode and one the anode. As the battery pushes electrons through the wiring from the anode to the cathode, things will be forced to happen at the anode and cathode. Namely, the atoms of nickel at the anode, which have been stripped of their electrons, become positively charged Ni+2 ions and dissolve into solution. Meanwhile positively charged Ni+2 ions in proximity to the cathode will receive those electrons and come out of solution as Nickel metal. So the electroplating process is moving metal from the anode to the cathode by moving the electrons through external wiring and causing ions to be oxidized into solution at the anode and reduced back to metal at the cathode.

There is a relationship here between the amount of current flowing and the amount of metal moved. For each two electrons you move, one atom moves. That's what we mean by 100% efficiency. Now say that you greatly increase the current flow and the plating rate shoots up -- so high that there aren't enough Ni+ ions right at the cathode to match all the electrons you are pumping over to it. What will happen is some of those electrons will start pulling H+ ions out of the water, converting them to hydrogen gas. If in this case say 10 percent of the electrons are going to liberate hydrogen instead of depositing nickel, the plating efficiency is now 90%.
As for greater than 100% efficiency, do you remember a science lab where you put an iron nail into copper sulphate ⇦ on eBay or Amazon [affil link] and it becomes plated with copper without any electricity applied? The difference in nobility between the cathode substrate and the metal in solution can cause metal deposition without external power being applied. So if you were to electroplate copper onto iron from a copper sulphate bath you could see more copper deposited than you can account for by counting the electrons you sent through the wiring, i.e., efficiency greater than 100 percent.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 12, 2011
Q. hello,
Where you state "There is a relationship here between the amount of current flowing and the amount of metal moved. For each two electrons you move, one atom moves. That's what we mean by 100% efficiency"
Is this true all the time, 2 electrons for one atom, or just this example?
Sorry if this is stupid - just trying to get my head around the basics at the moment.
kind regards
Tom
- Brighton
April 20, 2011
A. Hi, Tom.
That statement applied to nickel plating because nickel ionizes to a Ni+2 state, plus 2 electrons. Some other metals ionize to the +2 state, some metals ionize to the +1 state, some to the +3, +4, or +6 state. There may or may not be some that ionize to the +5 or +7 state -- I don't know offhand. Some, like copper, ionize to the +1 state in alkaline solutions and +2 state in acidic solutions.
If you look up the difference between a "molar" solution and a "normal" solution, it may become clearer to you that 96,485 coulombs (or one Faraday) of current will result in moving one gram equivalent weight of metal from the anode to the cathode (a gram equivalent weight is a molecular weight divided by the oxidation number of that metal, "2" in the case of nickel plating.
Chromium oxidizes in the "+6" state (hexavalent), and only plates at about 15% efficiency, so it takes a very lot of electricity to do heavy chrome plating.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
April 21, 2011
Q. I want more answer.
Abhi Nimbalkar- India
February 14, 2013
A. Hi Abhi. If you have a highly specific question, please detail it and we can attempt to answer it. If you mean you just want more general information, this is an electroplating book which will offer that .
Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q. What is the formula for calculating current efficiency for electroplating?
riyad siddique- motihar. rajshahi.bangladesh
November 16, 2015
Hi Riyad. Efficiency is this context would usually mean:
% = Actual weight of metal deposited / Theoretical weight predicted by Faraday's Law of Electrolysis.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
December 2015
March 26, 2016
Q. 1.CAN YOU PLEASE BRIEF EXACTLY AND TO THE POINT ABOUT THE CATHODE CURRENT EFFICIENCY BEING MORE THAN 100%.
2.AFTER HOW MUCH TIME OF OPERATION WE NEED TO CHANGE THE ELECTROLYTE SOLUTION?
3.WHAT ARE THE OPERATING VOLTAGE IN CASE OF
>COPPER PLATING
>NICKEL PLATING
>CHROMIUM PLATING
FOR BETTER CATHODE CURRENT EFFICIENCY. WHAT I MEANT IS, IS THERE ANY EFFECT OF VOLTAGE ON CATHODE CURRENT EFFICIENCY. SECONDLY DO WE NEED A MAINTAIN A MINIMUM OR MAXIMUM DIFFERENCE BETWEEN THE CURRENT AND VOLTAGE?
- HYDERABAD,TELANGANA, India
A. Hi Hareesh.
1. I just finished trying my best to do just exactly that in the 3rd paragraph of my first response :-(
Please express your ongoing question in terms of what was already offered so I can understand where the difficulty lies in your understanding of this.
2. In practical electroplating situations you almost never change the solutions; some shops have operated for decades without once dumping the contents. But please explain the details of what you are doing and we can suggest whether an adjustment or replacement of the solution is required.
3. The parameter which must be controlled in plating is amperage not voltage. Typical amperage for copper rack plating is probably about 30 ASF, for nickel plating about 40 ASF, for chromium plating about 150 ASF. The necessary voltage is Amperage * Resistance, and the resistance is primarily determined by the distance from the anodes to the cathode. Voltages tend to end up in the 6 - 12 volt range for copper and nickel rack plating. It's a little harder to say what the typical voltage for chromium plating is because it depends so heavily on the method of racking (conventional tank anodes vs. reversible rack system, etc.), but a voltage as low as 3 Volts or as high as 15-18 Volts probably isn't unusual.
It is best to carefully describe your own situation because, when you ask questions without doing so, we have no way of warning you about what might be important that you may have missed thinking about :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 2016
Q. Hi, I was just wondering, does cathodic efficiency decrease at higher temperatures? I was reading somewhere that this is true, and that at higher temperatures, the speed of formation of H2 increases more rapidly than the electrodeposition of metal. If this is/isn't true, can you please explain why?
Ashley Sanders- New York City, New York, United States
March 27, 2016
A. Hi Ashley. A very simple very general rule would seem to be that ions have more mobility at higher temperatures, so efficiency should be higher at higher temperatures. So I think I disagree with what you read "somewhere".
But please try your best to understand the thinking behind our guidelines re. not posting your questions in abstract fashion because it makes communication so difficult. We don't know if you're a 3rd grader or a post-doctorate ... I assume, but don't know, that your question regards electroplating, and from an aqueous solution, but even if so I don't know what metal you are electrodepositing. You are asking us to challenge something you saw in writing, but we have no idea who we are challenging, why, for what situation, or what limitations might apply to their claim :-)
Thanks & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
March 2016
Q. HELLO SIR,
FIRST OF ALL I WOULD LIKE TO THANK YOU FOR YOUR RESPONSE TO MY QUESTION.
ACTUALLY WE HAVE SOME ELECTROPLATING EQUIPMENT IN OUR LABORATORY IN WHICH WE NEED TO SET THE VOLTAGE AND CURRENT AND THEN START THE EXPERIMENT.
THE MAJOR PROBLEMS MANY OF US ARE FACING IS THAT THE CATHODE CURRENT EFFICIENCY IS VARYING WITH VOLTAGE, BUT ACCORDING TO THE FORMULA OF CATHODE CURRENT EFFICIENCY, THE WEIGHT OF METAL DEPOSITED DOES NOT DEPEND ON THE VOLTAGE BUT DEPENDS ONLY ON THE CURRENT. BESIDES, IN SOME CASES OUR CATHODE CURRENT EFFICIENCY IS DECREASING W.R.T TIME (here we are using the same solution for all time periods).
I AM NOT ABLE TO FIGURE OUT THE EXACT REASON BEHIND THESE TYPE OF TREND.(here we use the plates of dimensions 2X1 inch with thickness of about 2mm.)
- HYDERABAD,TELANGANA, India
March 27, 2016
March 2016
Thanks Haressh. Now that I understand that you are a student doing a laboratory exercise I can better understand your questions. Let me start over for you on how electroplating works. You put your cathode to be plated and your anodes which are the source of the plated metal into a salt of that metal, with wires attached to the anode and cathode. You connect your power supply. When "the current starts flowing", what we actually mean is that the power supply is pulling electrons from the anode and pumping them over to the cathode through the copper wiring. As electrons are thus stripped from the anode metal, it is being converted from atoms of metal to positively charged metallic ions. These ions dissolve into the plating solution and they migrate away from the anode and towards the cathode because the ions are positively charged and the anode is positively charged because it is now short of electrons, whereas the cathode is negatively charged because it now has a surplus of electrons. When the positively charged metallic ion reaches the cathode, it meets up with the necessary electrons to once again become an atom, and it deposits on the surface of the cathode.
Current or amperage is simply a measure of how many electrons you are moving. An ampere-second is one coulomb of electrons. It should be apparent that the number of electrons you move, i.e., the number of ampere-seconds, correlates with how many atoms of metal you ionize & dissolve at the anode, and reduce & deposit on the cathode. The relationship is called Faraday's Law: 96,485 ampere-seconds will ideally move one equivalent weight of metal.
Per Ohm's Law, E=IR or V=AR depending on what symbols you want to use. So we're not concerned about voltage except in as much as it causes the current to flow.
Now on to efficiency: We do not have a guarantee that when we move electrons from the anode to the cathode that the only possible thing that can happen is the conversion of metal ions into metal atoms. That's usually the dominant thing, of course, but something else that can happen is hydrogen can be liberated from the water instead. Just look at it this way: if there doesn't happen to be a metallic ion right next to the cathode when the electrons build up too much, the electrons will satisfy themselves by ripping hydrogen ions out of the water molecules instead:
2H+OH - + 2e - => H2^ + 2OH-
If you are not maintaining the solution concentration, and the metals are decreasing, the probability of a metallic ion being where it needs to be on the cathode at a given time decreases, so the efficiency decreases. Picture instantly turning the voltage or current up very high: there is no way a metal ion can instantaneously dissolve into solution at the anode and migrate over to the cathode, so you'll obviously pull hydrogen out of solution, decreasing the efficiency. As you increase the amperage or voltage the migrating ions have a bigger and bigger problem trying to keep up, and efficiency will start dropping when they can't keep up with the electrons.
Something which certainly could be happening to reduce your efficiency as time goes on is "anode polarization". This is a situation where, for one reason or another, the anode stops dissolving into solution. The solution therefore begins dropping in concentration, so the efficiency decreases.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
May 9, 2017
Q. Hi all,
I am writing to you in concern of hydrogen build-up and ignition over our alkaline zinc plating baths. I understand hydrogen can build up from the hydrolysis of water, but I am starting to question the electrolysis of caustic soda as well.
We run 3 plating lines. 4,500 gal tanks and only 2 of the tanks have this hydrogen build up. These two tanks also take 3x the amount of caustic soda ⇦liquid caustic soda in bulk on
Amazon [affil link]
.
All three plating baths plate at two different cathode positions with an anode on both sides. A-C-A A-C-A The only (Or highest) electric potential difference between the 2 hydrogen building plating baths and the plating bath is how they are bussed. None of the cathodes are bussed on both sides. On the two hydrogen build-up baths have both cathodes bussed on one side of the tank while the bath that does not build up hydrogen have one Cathode-Anode pair bussed on one side of the tank and the other pair bussed on the other.
Any suggestions/information on how to reduce or stop the occurrence and where the hydrogen is coming from? This will also greatly reduce my caustic soda consumption.
As for the electrolysis of water from poor efficiency, wouldn't this cause the solution to become more caustic and need less sodium hydroxide metering into the plating tank?
It is hard to determine the weight plated on a Hull Cell
⇦ huh?
. You need to strip the hull cell, dry it, re-pretreat and than plate and weigh. This seems like an inefficient method already.
- Indianapolis, Indiana, United State
Q. I am doing a lab experiment and it's about the efficiency of copper electroplating, and I chose to look at how voltage impacts the efficiency of the electroplating.
1. Firstly I want to know how voltage impacts the efficiency of the experiment. I am using voltages from 3 to 10 because apparently when you go above 10 volts the experiment won't work but I don't know why, and apparently, when the volts are at 3-6 it is the most efficient but I don't know why.
2. Another thing is that I want to calculate the percentage yield but I forgot to measure the current, but I measured the distance between the electrodes which was 6 cm and I know what the voltage was, how do I get the theoretical yield without the current?
- vienna, austria
November 18, 2017
A. Hi Omar.
1. Consider that a nickel-cadmium battery generates about 1-1/2 volts. If you tried to plate cadmium onto nickel and you applied less than 1-1/2 volts, current would be flowing in the wrong direction for any plating to occur. Thus there would be a minimum voltage required to do the plating of greater than 1-1/2 volts. If you tried plating at 2 volts, you'd have only 1/2 volt available to cover the resistance in the wiring and the solution, so plating would be achingly slow. Thus, something like 3 to 6 volts might be required to drive enough current to plate at a reasonable speed.
By the time you reached 10 volts, and probably long before that, you would be pumping electrons to the cathode so fast that metal ions would not be able to migrate through the solution and diffuse through the boundary layer fast enough to keep up with that surfeit of electrons, so some of the electrons would satisfy themselves by pulling charges from the hydrogen in water and releasing H2 gas instead of depositing metal. That's what we call inefficiency because the electrons you supplied are not all going to electroplating, but some are being wasted to the liberation of hydrogen.
2. A broken experiment is a broken experiment :-)
Unfortunately if you don't know the current, you can't know the efficiency. But I suppose you could try to look up the resistance of the plating solution you were using, and look up the electromotive potential difference between the substrate metal and the metal you deposited, and try to fake in the current readings based on your recorded voltages.
Hydrogen pops/explosions may be more dependent upon the ventilation/exhaust situation than the parameters of the solution. The lower explosive limit for hydrogen is about 4%; to my layman's brain that means that once other air mixes with the hydrogen at a 25:1 or higher ratio, it can no longer pop.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 2017
November 22, 2017
Hi Omar
Ted is correct but as a general comment, it is quite common to find that there is other chemistry going on in addition to the process you are studying. Faraday's laws are always obeyed but for example current used the release of hydrogen could lead to low cathode efficiency and occasionally autocatalytic reactions may look like efficiency over 100%. Even organic chemicals are subject to electrochemical reactions; typically oxidation.
You do not say what copper solution you are using but acid copper sulphate should obey Faraday's laws with no side reactions and can be assumed to be 100%. So much so that it has been used to calibrate ammeters.
With other copper processes and in the presence of other chemicals (e.g., brighteners) the above comments apply

Geoff Smith
Hampshire, England
Why chromium plating generates so much fumes
February 20, 2019Hi Ted,
Engineer Intern here looking for an better answer as to why chrome is emitted from some of these electroplating processes. I have a very basic understanding of the electroplating processes, so some of this might be ignorant.
Earlier in the forum, you wrote about how if no metallic ions are near the cathode, the electrons will pull hydrogen ions out the water molecule (where hydrogen at the cathode and oxygen at the anode gas are generated). I understand that some of the chromium (for example) will be entrained in the mists as the bubbles rise to the surface. To me, this means that there are plenty of metallic ions in the solution but just hadn't made the distance to the cathode before the gas evolves? Would this mean that the emissions would result (in part) by the distance between the cathode and anode?
Just looking for your thoughts on this. Thanks for your time.
- Tulsa, Oklahoma, USA
A. Hi Morgan. As you probably know better than me, every scientific explanation of how things work in our world is a simplification. Although many electroplating processes function generally as explained on this page, hexavalent chromium plating is a more mysterious process.
We know that there is a lot else going on besides ion transport, and that it doesn't work at all unless the solution contains a catalyst in almost exactly the right ratio (in the case of the Sergeant bath, sulfuric acid in the ratio of 1 part in 100), and that unlike some plating baths that can approach 100% efficiency, its efficiency is more like 12-15% and virtually never over 25%. So, regardless of chromium ions being near the cathode or not, or bath concentration, or anode-to-cathode distance, it always generates large amounts of hydrogen which fizz and entrain liquids which are largely chromic acid.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 2019
Q, A, or Comment on THIS thread -or- Start a NEW Thread
