
-----
Microscopic pores in galvanizing kettle due to lead
January 13, 2011
Dear readers,
Normally I use lead as bottom layer of the galvanizing kettle to avoid direct contact of dross with the floor of the kettle. But recently while starting a new plant in Saudi Arabia a German Gentleman recommend me not to use lead as it causes microscopic pores in the kettle, hence decreasing the life of the kettle.
Can anyone give me a brief explanation of this theory, as this concept is new to me.
Galvanizing Plant Employee - Pune, Maharashtra, India
January 13, 2011
Sir,
This theory is not correct.
Regards,
Galvanizing Consultant - Hot Springs, South Dakota, USA
May 4, 2011
Hello Dr.Cook,
Few days ago I asked a question regarding effect of lead on zinc kettle's life. Thanks for your answer. Well, within these days I had collected some more information about the same theory from a hand book prepared by Institute Stahlbau Leipzig GmbH and would like to know your opinion.
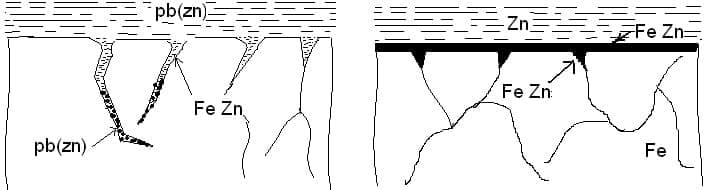
It is found that if the kettle is filled with both Zinc & Lead at the initial stage (heating up), some intercrystalline cracks develop during or shortly after the first time the zinc kettle is put into use. These brittle cracks, in some cases brittle rupture, occurs because of liquid metal corrosion (LMC) caused due to the intercrystalline attack by lead together with the tensile stresses in the kettle wall.
Hence a new kettle should be filled with zinc without lead so that the protective zinc-iron alloy layer can be formed. Lead should be added afterwards. The critical temperature whereby LMC comes into being raised without lead from 328° C ( the melting point of lead) to 420 ° C . LMC can only be formed when cracks are present in the steel, which are soon closed by the rapid formation of the zinc-iron alloy layer by the liquid zinc at 420 ° C, so that no intercrystalline cracks can be formed.
Regards,
- Jeddah, Kingdom of Saudi Arabia
May 20, 2011
Sir:
Thus the solution to your "problem" seems to be to add lead only after meltdown, which is easy to do. Actually I now have excellent galvanizing that is lead-free. In North America there is a move to go lead-free.
If you had told me that tin could cause these problems, then this idea is more understandable. Frankly in nearly 40 years I have never heard of a kettle failure at the bottom, except for one. This one was caused by a low temperature in the zinc and a very large pipe withdrawn nearly full of semi-frozen zinc and having the lifting chain break and the pipe plunge to the bottom and opening a hole in the bottom.
Galvanizing Consultant - Hot Springs, South Dakota, USA
Q, A, or Comment on THIS thread -or- Start a NEW Thread
