
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Can't electroform gold all the way into the corners
I am having a problem with cyanide based immersion gold plating. The parts are electrofomed on an aluminum mandrel using 99.99 percent pure copper. The aluminum is removed in a NaOH bath. During plating, I can not get the gold into the corners. In fact, I get a fine line, about 15 mil wide of white salt looking material. I tried removing the white material in an ultrasonic bath with water, then 10% by volume H2S04, both at 120F, neither affected the white material. I tried bleach at temp and most of the material was removed. Left behind was a trough of pure copper; the surface had been eaten into. The gold on the flat surfaces was also damaged by the bleach though it was exposed for only 13 min.
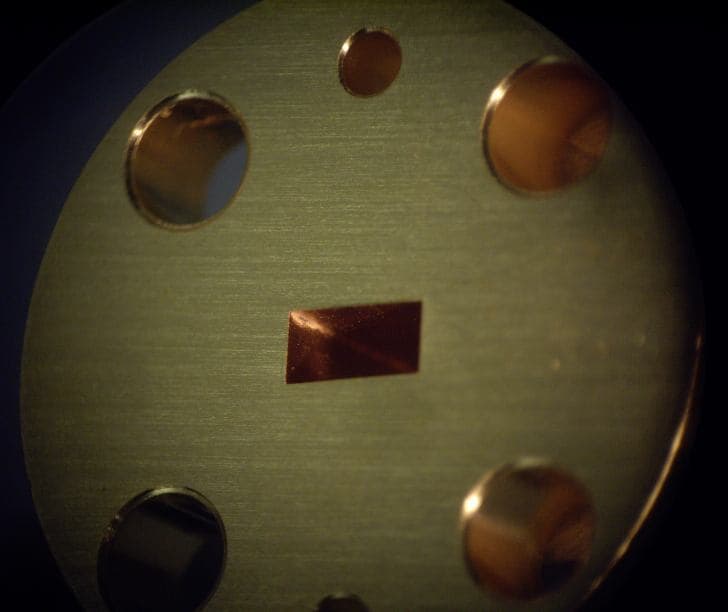
What is this white salt like material, and how does one get good plating into the corners?
Sincerely,
Gary
Gary SalvailMarch 11, 2010
April 22, 2010
The ability of a plating solution to deposit into corners is called the throwing power.
Many gold processes have poor throwing power but some are specifically formulated to enhance this e.g., sulphite based solutions.
The best way forward is to speak to your gold supplier.
Don't forget that your current process could be very useful for those times you do not want gold in the holes - it could save you having to mask them.

Geoff Smith
Hampshire, England
I'm a little confused here. Gary says he's having trouble with his cyanide based immersion gold process. So which is it, electroplating, electroforming or immersion gold?
Mark Bakerprocess engineer - Malone, New York
April 22, 2010
Q, A, or Comment on THIS thread -or- Start a NEW Thread