
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Electropolishing of stainless steel -- Q&A, Problems & solutions
Q. I have a 316 stainless steel tank with an attached funnel which is used to store alcohol based solution. The solution is changed every 5 to 10 days and the tank is washed thoroughly before a new solution is stored inside. The only surface finishing on the tank is 2B finishing and electropolishing. Recently, I have noticed the inner wall of the funnel has started peeling off. Please see attached picture.



I will greatly appreciate your help in identifying possible causes of the peeling. Thank you.
S Choice- Sacramento, California
May 23, 2024
A. Choice,
The only plausible way for the surface to be "peeling" is if there is some kind of coating or residue there. Perhaps whatever is in the alcohol solution being poured through this funnel, being left behind on the surface as the alcohol evaporates.

Ray Kremer
Stellar Solutions, Inc.
McHenry, Illinois

|
|
Q. Hello Ray, - Sacramento, California A. Choice, - Ballarat, Australia May 29, 2024 |
⇩ Related postings, oldest first ⇩
Q. My name's Nick Matthews and I would appreciate some help on the following problem we are experiencing:
We have our stainless steel (316L) tube units (vary in diameter between 600 and 1600 mm in diameter) electro-polished by a sub-contractor. Occasionally we observe small imperfections on the electro-polished tube wall which can be best described as 'comets'. i.e. they have a small head from which emanates a straight 'tail'. The head is approx. 0.2mm and the tail is typically 5mm in length. The tails are all in the same direction, as if they are 'escaping' from the surface and rising to the surface of the electro-polishing tank.
Has anyone else experienced this problem and more importantly identified the cause ?
Thanks
Nick
- Glos, UK
2000
and Chemical Polishing
of Metals in
Research and Industry"
by W.J. Tegart

on eBay or
AbeBooks
or Amazon
(affil links)
A. This is book knowledge rather than 'hands-on', but I think this may what is called "gas streaking" due to excessive current density in that area. Certainly a closer look would be needed though before insisting that this rather than poor mechanical polishing is the real culprit.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. Dear Nick, I used to run a lot of Electropolish of 304 303 types of stainless, our problem was much as you're talking, In that scenario, although the Manufacturer was buying 304 /303 type of stainless it was not "pure" enough (back then they called it electropolish grade(, we sometime would get these "comets" in straight streaks or misc depending on how this material was formed. The reason for the "tails" the pit or head of the "comet" is were the contaminate was at its most concentration and did not mix thoroughly
Chris Snyderplater - Charlotte, North Carolina
A. Hi Nick,
It seems that due to a locally high concentration of one of the SS316L elements, that area is more active than the other. The comet shape you described is a result of gas bubbles formed at the active area.
You may try to reduce voltage (current) or circulate the acid so gas bubbles will evacuate rapidly from surface.
By the way, which polishing acid do you use?
Oded Nissan- Neve Ilan, ISRAEL
Q. Hi. My name is Jacqueline Tong. I was reading the earlier message, and I have the same problem. We electropolish stainless steel 316L implant plates, and occasionally we get straight scratches or water stains-like marks on the surfaces. The problem seems to show up at random.
I am trying gather information on all possible causes:
1. If the problem is due to 'gas streaking', how should we correct this?
2. If it is due to contaminants or inclusions in the metal, how can they be identified.
3. Can waxy residues from previous surfaces treatments cause the problem?
4. Can other environmental factors, such as temperature, humidity, etc., be the cause?
Any help will be greatly appreciated. Thanks.
Jacqueline Tong- Canada
2000
Q. Sorry,
I'm not able to help with the above problem, but I have a similar issue. We are electropolishing 316L Stainless steel. Occasionally we find a 'dry grey' contamination on the surface of the steel. It seems to be a mixture of phosphates and sulphates. Just wondering if anyone else has experienced this problem. If so, how can it be fixed?
Ivan Mooney- Ireland
2000
A. To Jacqueline Tong and Ivan Mooney:
Electropolishing defects can be caused by a wide variety of problems in the substrate, the preparatory processes, and in the electropolishing procedures. It is generally not possible to pinpoint the cause of any specific complaint by remote control.
Regards,
Ed BayhaMetal Coating Process Corporation - Charlotte, North Carolina
![]() Jacqueline, Ivan,
Jacqueline, Ivan,
Mr. Bayha is surely correct that it can be tough to troubleshoot a process remotely, and his implication that one should get some level of actual targeted training before they will be able troubleshoot effectively is a great point too!
Still ... the same can be said about every metal finishing process which we discuss here. So after years of postings, and receiving hundreds of letters of thanks, we're certainly not about to to concede that posting trouble-shooting questions here is futile :-)
Most of the "must have" texts have pretty good introductions to electropolishing of stainless steel. The Electroplating Engineering Handbook is perhaps the most thorough with a chapter of over 20 pages ⇨
The AESF has an excellent illustrated lecture (slides & text) on electropolishing.
If you can send along some photos you can probably get some targeted responses. For example:
• An evidence of gas streaking is the path of the disturbance or "streak" compared to the way the part is racked.
• An evidence of contaminants would be their location relevant to the machining operations.
• An evidence of waxy residues would be a water break before electropolishing. Poor rinsing could leave grey salts on the work.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Multiple threads merged: please forgive chronology errors and repetition 🙂
Problem with Electropolishing SS410 material
Q. Dear all,
I'm a process engineer in a plating house in Singapore. Our original process is capable of electropolishing SS300 series materials. But recently our customer want to do EP on SS400 material(micro clamp). These micro clamp after EP turns black. Can anyone share your experience with me if you have done success EP on similar materials and parts?
Our current EP setting is: %80 phosphoric acid, 15% ethylene glycol, and 5% tributylamine, at 60 °C rotation for 2 minutes. Our bath change frequency is 1 week or max 2 week, and phosphoric acid will be toped up everyday.( For voltage, I tried 2 volt to 6 volt, it seems higher voltage causes blacker surface. But I don think lower voltage is good, as it does not really get the expected cleaning effect).
Another problem with this part: as the part is very small and very easy to adhesive to each other, so the overlapping problem is very serious. After we used some dummy to separate them, the result is still not satisfactory. We don't have the condition to do jigging process, and I already adjust the barrel rotation speed to max. Is there any other condition I can change to improve the overlapping problem?
Your suggestions will be greatly appreciated, and I will share with you any improvement on my side.
Electropolish, plating house - Singapore
2005
Multiple threads merged: please forgive chronology errors and repetition 🙂
Q. We are a manufacturer of stainless steel (17-4 PH and 455) instruments for orthopedic industry. We are trying to get a smooth and even surface finish on stainless steel parts after electropolishing. Our current sequence of process is Machine, Heat Treat, Clean, Glass bead and Electropolish. This sequence is causing surface unevenness and staining problems.
I spoke to a lot of suppliers and they gave me feedback that this may be because of residual heat treat scales and uneven glass bead surface. The media that we use for glass bead is glass silica with size of 100-150 microns.
Can someone please give me a feedback if the root cause is the glass bead media/process or uneven residual heat treat scales?
Is there any solution to obtain a smooth and even surface finish after glass bead and EP? PS: We do not want to change the sequence to EP and then glass bead.
Any kind of suggestions will be appreciated.
Thanks
Quality Engineer - Piscataway, New Jersey
November 18, 2008
|
A. Divya - Colorado Springs, Colorado A. Divya, I'd look at both areas you mention. If your company performs both heat treat and blast, you may have more direct control over ruling one or the other out. Glass Bead blasting is straight forward, but several parameters should be noted: Media size and conformance to spec, working mix "beads in blaster" conformance to media spec, pressure, distance to part, nozzle, time, classifier performance if reusing media, etc...also you may find that many "offshore" glass medias have poor life in blast units and breakdown prematurely leaving inconsistent cleaning results. Regards, Tim DeakinNorth Tonawanda, New York |
A. Maybe your glass beads are creating a compression stresses, which causes more active sites in your metal.
Try changing blasting angle or better to change to diffused fine aluminum oxide.
Good luck.
P.S. please feedback if it does help!

Khair Shishani
aircraft maintenance - Al Ain, UAE
A. I do not have any direct experience of electropolishing surfaces blasted with glass beads. However surfaces with heat treatment oxide are not electropolished well, the surface ends up matt and blotchy.
The oxide may be removed chemically. A solution of 100 gms/l sodium hydroxide and up to 100 gms/l potassium permanganate
⇦ this on
eBay or
Amazon [affil links] at 90 °C for 1 to 3 hours degrades the oxide. Afterwards the work usually has a thick brown layer (containing manganese dioxide) which must be removed in inhibited hydrochloric acid. This process might have to be repeated to remove all the heat treatment oxide.
If the glass beads are pushing oxide into the surface I doubt whether electropolishing stands any chance of success.
- Whitstable, Kent, UK
A. Try adding an initial blast with 120-240 mesh aluminum oxide. This will remove residual oxides from HT and will leave a surface that is easier to inspect visually for uniformity before going to the glass beads, specially for intricate parts or not-so-skilled operators.
Caution: this step will remove some metal, so, parts with very tight tolerances may not be suitable.
Monterrey, NL, Mexico
adv.
Send us a few parts after heat treatment. Our electrolyte does not require oxide removal before electropolishing.

Anna Berkovich
Russamer Lab
Pittsburgh, Pennsylvania

January 6, 2009
Q. Last 3 weeks I have a problem to electropolish straight surfaces.
I get stains on the surface.
It's hard to see that on pictures, but I marked the stains as good as I could.
What can I do?

- israel
June 25, 2019
Shadows in electropolishing of 304 SS
Q. Hello,
I've encountered a problem in electropolishing of bowl-like pressed stainless steel sheets.
The bowl is 25cm in diameter and has 12mm hole in center by which it is fixed on Ti rack.
Inflexion of the bowl is about 4cm.(see photo)

Above the fixture in the inner part of the bowl, is a shadow after electropolishing. I found out by measuring the current in that area that there is about 1/3 of the current density then elsewhere, which is why it probably leaves shadows.
Distance between electrodes is 12 cm on both sides.
I've tried stirring the solution with bubbling, but it does not help much, probably because bubbles can't reach inner part of the bowl.
Bowl is fixed vertically, as both sides need to be electropolished.
Part is etched and cleaned before polishing.
My thought is that the H bubbles from anode are decreasing conductivity, so current is lower than needed.
Still, it is in polishing zone, as polishing it for longer time (about 2 times as long) removes the shadow.
Parameters of the process:
Bowl surface 9dm2, Current per part 60A -> 6.6 A/dm2
sulfuric (96%)-phosphoric acid (85%) 33:67, plus added glycerine 5%. Mixed it few days ago, so water is not a problem.
Anyone has any idea how to fix that?
Funny part is that old electrolyte did not leave shadows, but it is more than 2 years ago since I mixed it and can't remember the composition I used.
Btw. given electrolyte has minimal polishing current of 0.8 A/dm2, which is the best I've ever mixed. Though it's a bit slower polishing than more sulfuric mixtures.
To simplify the question: Is there any way to improve polishing "depth" without adding helper cathodes?
- Brno, Czech Republic
March 25, 2020
Electropolishing 303 SS
Q. My name is Patrick and I had some large 304 SS parts bead blasted to remove pitted rust. They are now being electropolished but are showing signs of inconsistent finish as well as a light layer of black stuff. Attached is a couple of pictures.



This can be wiped off with a paper towel but I am being told it is a result of the blasting process. Any thoughts on how to prevent or how to clean?
Patrick Daniel- Dothan, Alabama
August 25, 2020
Q. Last 2 weeks I have a problem to electropolish surfaces, like a blister defects, but roughness is normal.

What can I do? Material ss 316
KALYAN DUTTA- Pune India
September 11, 2020
A. KLAYAN DATTA,
MAYBE YOUR CHEMICAL ELECTROLYTE BATH, IRON % IS TOO HIGH.
- BHARUCH, GUJRAT, India
Q. We are a metal forging company since 1940's. We have been forging 316 SS for an extensive time. Recently, we are having parts returned for black spots appearing after the electropolishing process. How can we tell if the electropolishing process is causing the issue or if it's our forging process? What possible issues can cause these black blemishes and how can we find the root cause?
Lisa Venette- Tonawanda New York
September 30, 2020
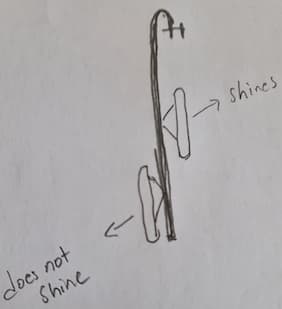
Q. Hi,
When we do electropolishing with the hanger, the specimen on the upper part of the hanger shines; the specimen at the bottom does not shine. Can you please help me with this situation?
Thank you for your suggestions.
Omar Muhammad- London U.K.
August 1, 2021
A. Omar,
Electropolishing is a line of sight process, so if you don't have direct sight of the cathode the part doesn't electropolish very well or one part is closer to the cathode than the other which would also give you two different results. Large parts you need more than one contact point on the part, so the current flows evenly through the part.

Mark Battles
Plymouth, Minnesota
Dark frosty cloud dull finish in electropolishing!
Q. I am electropolishing Rectangular ss tank having 140 mm depth with the help of auxilliary lead cathode but mirror finish electropolishing is not commencing inside all the surface.

Can anybody help me?
SUNIL KUMAR GARGF- GHAZIABAD UTTARPARDESH INDIA
July 3, 2022
⇦ Tip: Readers want to learn from Your Situation 🙂
many readers skip abstract questions.
Q. How to EP inside a long tube? And how to calculate surface of anode (inside surface of tube or inside + outside surface of tube)?
Thank you.
hobbyist - Bangkok Thailand
February 26, 2023
A. Hi Aek. The Electroplating Engineering Handbook says: "Whenever possible, cathode to part ratio should be about 2:1."
Obviously this is an unlikelihood for an internal anode inside a tube, but as large as practical seems like a good idea. They probably need to be racked vertically. Agitation inside the tube is probably necessary depending on how long is "long".
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread

