
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Tin Plated Parts are Tarnishing Black
this text gets replaced with bannerText

Q. Hello.
I work in the electrical/manufacturing industry, and often come across electrical terminals that have plating that doesn't seem to look very good.
Functionally they don't cause any issue as far as I can tell, but the look of the terminals always raises the question 'are they ok?'
The case in point in the image is that the 2 terminals shown come from the same reel of AMP 150530-1 terminals. One looks completely fine, the tin plating is uniform, but the other appears tarnished with a mottled finish.

We just buy the reels from the manufacturer, with the understanding that the reel conforms to the quality criteria of the manufacturer. These are stored on a shelf until they are needed.
I guess my questions are;
1. What causes the difference in finish?
2. Are the tarnished terminals ok to use?
3. what are the long term effects of the tarnish?
Thanks.
- Melbourne, Victoria, Australia
September 25, 2023
Q. Hi sir/madam;
Recently we have customer complaint regarding our part.
Our part's base material is SUS 304 1/2H with Pre-selective Tin plate min. 3.5µm (both sides), Ni under-layer (0.50~1.5µm).
They have observed that our part's tin plated area turns out to be a little yellowish. After they do soldering, the color of soldering tin has changed to black on the part itself. Do you have any idea why it has changed to black? Is the yellowish color of tin plating area one factor that causes black after soldering. Your reply is greatly appreciated.
Regards,
TOM
- SINGAPORE
November 10, 2023
⇩ Related postings, oldest first ⇩
Q. We are using many Tin plated brass components in our product.We are facing a problem of oxidation of this parts. This parts oxidize within 2-3 months while storing. The surface of this parts becomes black. Tin plating of the part is our customer requirement so we can not change the plating. Would someone will give any suggestion to overcome this problem Thanks in advance.
Bahadur Singh Rathore- Udaipur, Raj., India
2000
A. Bahadur, Without seeing the parts it sounds like de-zincification of the brass. With zinc being anodic towards tin it will tend to migrate into the tin deposit over a period of time and cause the discoloration you are seeing. You will need a copper or nickel barrier layer to prevent this.
Rick Richardson, MSFDayton, Ohio
A. Black on tin plate is almost always caused by the sulfur in cheap paper vaporizing and tarnishing the tin. Wrap in the fine white paper that they use in department stores that sell silver and fine china. for longer term storage in a non air conditioned environment, store in plastic boxes--Tupperware--type in the USA.
For oxidation, we used to dip the parts in a solution of stearic acid
⇦ this on
eBay or
Amazon [affil links] dissolved in Xylene
⇦ this on
eBay
or
Amazon [affil links] . this was per some specification that I do not remember the name. Probably military or ASM. This will about double the shelf life of the solderability of parts.
- Navarre, Florida
A. You need a copper layer between the brass and the tin to avoid migration
You also need a mild alkaline post dip
On top of that, if the solution is old it accumulates organic breakdown products. In this case you have to dilute or dump the tin plating solution

Sara Michaeli
Tel-Aviv-Yafo, Israel
February 8, 2010
A. This issue could happen on brass or other alloy contains zinc, because during the dipping process brass could easily form zinc-concentrated areas, which will restore the acid solution (sulfuric acid) back to sulfur during the plating process. The sulfur will gradually react with tin and form tin monosulfide which appears in dark color.
Pre-coating with copper for isolation between zinc and tin could prevent this issue.
- Xiamen, Fujian, China
November 13, 2011
Q. We use a component with two terminals, the terminals have tin plate coating. At the end of my process the terminals have a oxide layer, How can I remove this oxide layer?
Oswaldo Montes Palmaproduct designer - Matamoros, Tamaulipas, Mexico
2007
A. Hello, Oswaldo, welcome to finishing.com. I don't think that is the ideal question though! In my opinion what you should do is prevent the tarnishing by applying a tarnish banning post treatment after the tin plating, and by using procedures that will eliminate staining, including operators wearing gloves and never touching the terminals, tarnish preventative packaging, and minimizing the time the parts are in process.
Another possibility is to use electroless nickel plating instead of tin. Many if not most 'charging' contacts on cordless phones, cell phones, etc., are electroless nickel plated.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Black marks on tin plated copper
Q. Very good morning to you all,
Actually these day we are facing black mark problem on tin plating surface in one of our products parts. Do any have an idea to clean that black mark problem? It's urgent -- please advise. We have tin plating thickness of 4-7 micron on copper.
engineer - Haridwar, India
September 26, 2010
Tin plated brass turned completely dark black
Q. I have terminals that have turned completely and uniformly black. Brass base material is 70% Cu, 30% Zn. Plating is a 4µ Cu flash with 8µ Sn.
I put 500 pieces from a 1000 piece bag in a bowl feeder with a clear plastic cover in our production line on Friday. They sat over the weekend under climate controlled conditions. They were all black on Monday. The remaining parts in the bag on the shelf were still Sn color.
We have run this process in this manner for many months with no issue. The plating supplier has never seen this issue. We cut and sectioned a part to confirm the Cu layer.
Is there any agents, chemical or otherwise, that would activate this process? Is the black layer an oxidation?
Our manufacturing area is very tightly controlled for contamination.
Any information is appreciated.

Thank you,
Steve Pugh- Anderson, South Carolina, USA
April 30, 2012
A. Hi Steve.
"When you've ruled out the impossible, then, no matter how improbable, . . ."
Tin plated parts are subject to a darkening condition called fretting corrosion. Any chance that somebody accidentally left that vibrator on over the weekend?
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
![]() Hello Ted,
Hello Ted,
Our operator advised that the vibratory bowl and equipment was all powered down over the weekend so we ruled that out at first. We have done re-creation tests. We are able to recreate the problem by leaving the vibratory bowl on for 48 hours. Somewhere along the line it was left on. The answer to your question is yes!
Thank you,
- Anderson, South Carolina, USA
Tin plated meat grinders turn black
Q. Why do tin coated cast iron meat grinders turn black? The better quality hand powered cast iron meat and all purpose grinders have traditionally always been hot dip tin coated. This includes the ones still made today in the orient and in Europe.
It is very common for dark spots to develop over the years until only a little bit of shiny tin is visible. I have always assumed the dark stuff was rust and the areas where it occurred were the spots where the tin had worn completely away, but the dark spots resemble the iron oxide one gets when one treats rust with phosphoric acid, which has me wondering if it isn't the tin itself, which has for some reason changed color. Also, I notice some areas where the tin has clearly been completely removed have normal reddish rust. Like iron oxide, this black stuff is very hard, and using Scotchbrite I have had no luck removing it (I don't want to use anything harder, for fear of damaging whatever tin is left),
The dark spots are sometimes a bit greasy feeling, and bead water, and other times dry, so dry that tiny chips will fall off on your hands if you rub it.
Anyone have any idea what the black stuff is, and whether there is any way to remove it or recover some of the tin that may lie beneath?
- Concord, New Hampshire, USA
November 4, 2012
Tin plating turns black after a few months
Q. Dear Sir,
I'm facing tin plating problems.
We do bright acid tin plating on copper lugs. For some reason they are turning black within 4-5 months.
We got a customer complaint of the material dispatched in August 2012
Please help me.
Thanks
November 5, 2012
Engineer - India
A. Hi Jack,
The problem could be due to tin tarnishing upon long storage and/or harsh storage environment (high temp high humidity, corrosive environment, etc). You may apply proprietary post dip (other than generic trisodium phosphate
⇦ this on
eBay
or
Amazon [affil links]
) after tin plating to create an hydrophobic protective layer on top of tin deposit against air/moisture contact.
Regards,
David

David Shiu
- Singapore
A. Hi,
It's a migration. Use a nickel strike instead of copper.
Regards

Anders Sundman
4th Generation Surface Engineering
Consultant - Arvika,
Sweden
Q. Dear All,
We are doing bright acid tin on copper lugs and this are d results we are facing after certain period of time.
Can any one help me to solve this problem as this is affecting me to loss of costumers?

Engineer - Mumbai, Maharahstra, India
April 4, 2013
A. Abhiraj,
It sounds like you're getting SnO or SnO2 forming from slow interaction between the tin plating and cleaning agents with a high pH (heavy in the OH group). This means that the plating was doing exactly what it was supposed to do, oxidizing so the metal underneath did not.
To sum up:
Hot + humid + high pH = blue black SnO
If it's gone too far you may want to get them re-plated.
I don't think that recovering the tin from the plating is going to be cost or time effective. It's unlikely that you have more than 3 g of tin plated on any one grinder. Recovery would be a multi-step process involving various acids, reagents, and processing probably exceeding the cost of purchasing tin metal.
Ref: .
Blacksmith - Boone, North Carolina, USA
A. Hi,
It also could be tin plague. A tin deposit with no alloy in the tin deposit change from Alpha phase to beta phase in cold atmosphere and will dissolve.
Regards

Anders Sundman
4th Generation Surface Engineering
Consultant - Arvika,
Sweden
Q. Thank you all for your valuable response on my email.
Can you please suggest remedies for such problems that they shouldn't occur again after tin plating?
- Mumbai, Maharashtra, India
April 12, 2013
A. Abhiraj,
If the parts are frequently exposed to moisture and aggressive cleaning agents I'd suggest paint or enamel. There are several 2 part epoxy paints that would probably do quite well for providing an additional moisture barrier.
Blacksmith - Boone, North Carolina, USA
A. Anders has a good point - you may need a barrier layer, such as nickel. However, I reckon the tin has migrated into the copper and formed an intermetallic compound (Cu3Sn). This can be a very fast process and will increase in rate at elevated temperatures. The inclusion of a barrier layer will stop it.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Q. We are currently experiencing uneven and low thickness and rough deposit of tin plating on Cu. Our additive is below the specs (122 ml/l target, actual 95 ml/l) for we have issue on out of stock primary rbm additive. In addition, we also observed Nickel on tin deposit from EDS analysis. Do you have any suggestion on containment action that we can do while waiting for the additive delivery? Also, how can we remove Nickel from tin plating bath if it will be proven at high ppm level? Thanks for your time and looking get your expertise regarding our concern.
Trisha Reyes- Laguna, Phils
April 23, 2013
Q. Hello,
We make copper and brass parts for transformers. The brass used is 56 Cu, which is diecast to form components. The machined components are tin plated, sometimes with a pre coat of copper and otherwise with nickel. The parts are turning dark within as less as 2 days. The transformers are used in heavily polluted atmospheres.
Please advise on the coating thickness of tin and copper/nickel to prevent this, also if it is happening because of partly rough surface of the machined component?
- New Delhi, DELHI, India
July 12, 2013
Q. We are using some copper bus bars with nickel undercoat and top matte tin plating. In one batch we found that plated parts are getting blackish. I want to know the reason behind this issue.
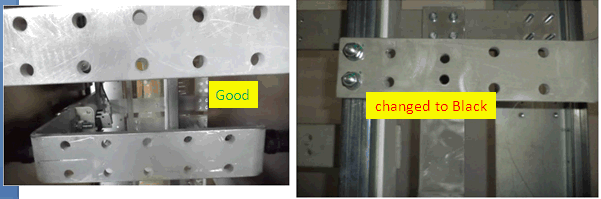


Raghava Reddy
- Bangalore, India
August 26, 2013
A. Hi Raghava. We appended your question to a thread which offers food for thought. There are many possible causes of tin blackening, from sulfur in the air or in the packaging, to fretting corrosion, to contamination of the plating bath, etc. We'd like to help you, but it is not easily dispatched without a lot of operating data. The plastic film and cardboard packaging is certainly a prime suspect. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Hi Ted, can you explain more why plastic film and the cardboard become the main suspect for the blackening happened?
Josafat Malau- Indonesia, East Java
September 5, 2024
A. Hi Josafat,
Sulfur in paper or cardboard packaging is a common causes of blackening as James Watts described; other readers followed up with detailed responses explaining it and offering alternate theses. Sealing dampness into plastic is a common corrosion cause of corrosion, but I didn't say that it was the main suspect.
Please give us the details of your situation rather than asking readers to conjecture about possible relationships between your unstated problem to someone's else's problem from a decade ago, which was offered with no data either, and where they might have been using cardboard or might have been using plastic or neither ... and where we'll never know whether our answers proved helpful or not :-)
When you want feedback about a specific problem which you are encountering and offer data about it, readers often enjoy trying to help. If you disclose nothing, so they will learn nothing from their efforts, they usually don't bother replying.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Tin Plated Parts are Turning Black
We are using tin plating for aluminium components. We have a oxidation or immigration, the surface of the parts are coming black. The parts are in storage for 3 to 5 months. Would someone will give any suggestion to overcome this problem Thanks in advance.
- Juarez, Chihuahua, Mexico
August 28, 2013
A. Alkaline stannate tin brighteners will not "rinse" off, you must go back into a mild soak cleaner to remove the brightener film - if left on, the tin deposit turns black.

Robert H Probert
Robert H Probert Technical Services
Garner, North Carolina

Q. Finish of aluminium bus bar with nickel undercoat and top matte tin coated parts color differs from batch to batch and some parts looks like black instead of light grey.
Why? And how to control this?
How can we get uniform color in this kind of plating ?

Raghava Reddy [returning]
- Bangalore, India
August 30, 2013
A. Hi Raghava,
The black colour could be due to poor tin grain structure (e.g. burnt deposit at high current density) or tin oxidation.
To get uniform tin deposit colour, you should follow supplier recommendation to analyse and replenish proprietary additives (e.g. leveler, grain refiner, etc) and to insert post treatment (e.g. neutralizer, anti-tarnish) and adequate DI water rinses after tin plating to ensure plating chemical residues are fully removed prior drying and tin deposit is protected from oxidation by anti-tarnish coating (hydrophobic coating).
Regards,
David

David Shiu
- Singapore
⇦ Tip: Readers want to learn from Your Situation 🙂
many readers skip abstract questions.
Q. Hi,
We are also facing same issue, did you got the root cause?
- Khopoli, India
November 3, 2022
A. Hi Lokesh. Raghava did not respond to the suggestions sent to him, and I doubt that we'll be able to reach him almost 10 years later although we'll try. But there are so many possible causes for blackening of tin discussed on this page that the cause of the blackening on your nickel plated and tin plated aluminum bus bar may not be the same as his anyway.
But is there a particular reason you are adding the underlayer of nickel? The simplest way to plate aluminum bus bar is probably to use an immersion stannate followed by tin electroplating. Atotech called this process Alstan, and they probably have competitors offering a similar approach, and many shops do it.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Hello all,
I've got the same problem with a Zinc diecast product which is plated with Tin over Nickel over Copper.
We use a reflow temperature of 240 °C.
After reflow about 40% of the parts turned brown.
If I want to put an anti-tarnish layer on, which layer I can choose as the best? Do you have brands and types?
- Hertogenbosch, The Netherlands
November 27, 2013
A. Gerard, Tin will form intermetallic compounds with copper, and nickel and I believe it will quickly diffuse into zinc. I believe that the interaction of tin on zinc is a way of producing tin and zinc whiskers at very fast rates, so there is certainly some adverse interactions going on. The rate of formation of the Cu-Sn intermetallic compounds is much faster than those for Ni-Sn and since you are flow melting at 240 °C - above the melting point of tin, the diffusion rates are significantly enhanced. I suggest you increase the amount of tin put down and reduce the time the substrate is held at an elevated temperature.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
Q. Hello Trevor,
Thanks for your quick response; indeed that is the reason we put first copper and then nickel as barrier between Zinc and Tin. Maybe increasing the tin layer is a suitable solution. Actually we are starting a trail run with a anti-tarnish layer after the plating operation. For your information, the connector is soldered by reflow which means that the max. temp of 240 °C is only a couple of seconds. I'll keep you informed about the test results if you are interested?
- Hertogenbosch, Netherlands
December 9, 2013
Tin plated pistons have black stains from storage
Q. Hi
Recently, I found that all the tin-plated (automotive) pistons stored in my warehouse have strange black stains. It appears as if they were all dipped in some solution and then left to allow dripping and drying.
Can anybody suggest me some remedy otherwise, all that huge stock will be a big blow for me.
Thanks in advance for the help.
- Lahore, Pakistan
July 20, 2014
Q. We are producing tin plated copper bus bar but I have problem that some areas turn black maybe after one day.
Last time we sent to customer he sent a complaint, as we do tin coating, and in next day we send it to customer: BUT WHEN HE OPENS THE BOX HE FOUND BLACK SPOTS ON COPPER BAR. So please anyone can guide me what to do?
- riyadh in saudi arabia
February 8, 2015
A. Hi Ahmed. You posted your inquiry onto a thread with lots of hints about possible causes. But my first guess is that James Watts is right, that you are not using proper sulfur-free packaging. If you are, please try to frame your ongoing questions in terms of the suggestions that have been offered. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Tin plated (min. 5 µ) Al parts surface going dark in very short period; tarnishing starts in just 1-2 weeks. Process steps: Zincate + Cyanide Cu (max. 1 µ) + Tin (min. 5 µ)
Any suggestions about this problem? Many many thanks.
- istanbul-Turkey
February 20, 2015
Removing stannic ion fixed tin plating that was turning black, but now the bath is blue
A. Hi last time I used phosphoric acid to precipitate all the stannic tin which causes the pieces to turn black ...
Q. Now I have good results but the color of the bath turned from yellow to blue -- is that normal?
capacho franciscoIngezinc. - bogota,Colombia
May 18, 2015
Unknown Black Deposit on Tin Plated Copper Brazed Joint
Q. We have a problem with an unknown black deposit only occurring on the bottom joint from where the part is hung when tin plated. All of the other sides of the joint are fine.This occurred equally on 5 joints that are on this part. It is a copper bus bar with BAg-7 braze alloy that was torch brazed. We have also seen this occur on similar products when resistance welded, using BAg-7. All parts have been quenched using an Isoprep 192 solution, rinsed in water, and dried.
The deposit is relatively easy to remove, but underneath is bare copper. We currently use a wire wheel to remove it and paddle plate the affected area with no problem.
BAg-7 Alloy is 56% Ag, 22% Cu, 5% Sn, and 17% Zn
Flux Used is Silvaloy Black Flux for Silver Brazing
When able I will add pictures.
- Monee
October 21, 2015
Q. Hi there, I seem to be having a few issues with my tin plating process
1) What is the correct temperature to do the tin plating at?
2) Why is it that when the amperage is high, the plating tank/bath seems to create a lot of foam on the surface?
3) I am getting black deposits on the items being plated, is there any way to remedy this?
4) I have used chemicals from one supplier, however I have changed suppliers but the chemicals they use for tin plating is different, how would it affect the tank composition/chemical balance?
5) Our tanks were initially made up of only Atotech's chemicals, however they no longer have a branch in South Africa. Is there anyway that I can import the chemicals or find another distributor of Atotech's chemicals?
Thank you for your input, It will be much appreciated
- Cape Town, South Africa
October 2, 2016
A. Hi Paul. We appended your inquiry to a thread listing some of the causes of tin plating turning black. As you can see, there are a lot of possibilities, some of which depend on the substrate and the type of bath.
Help us envision the situation by telling us whether you are rack, barrel, or strip plating; what metal the substrate is; the purpose of the plating (electronic parts, decorative, etc.); and what type of plating bath you are operating (MSA, bright acid tin, stannate). Thanks!
Mixing chemicals from two suppliers is a problem. I think you should find a distributor of tin plating processes in South Africa. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Why black color marks are formed on the tin coated bus bars of the new panel board which is not yet energized?
irshad alamabc - riyadh, saudi arabia
April 24, 2018
Q. My situation:HI!
I am a Plater. While doing tin plating, I am facing problem of blackness on brass terminal during Tin plating after tinning of 1-2 days.
Please give solution.
Plater/Businessman - Gurgaon,Haryana/India
July 19, 2018
A. Hi Irshad, Hi Repender. As you see, we appended your inquiries to one of several threads about blackening of tin plating. Please review the page and then ask any questions or explain the ways in which your situation might be different from what has been described. Thanks!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. Dear Rupender,
The blackness over tin plating is called tarnish on the plated surface. After tin plating the parts must be neutralised by mild alkaline solution then good rinsing.
Hope it will help you,
Thanks
- Electronics City, Bangalore, India
Tin plated copper electrical work turning jet black
Q. Hi, we provide electrical engineering support to a nickel concentrate plant supplied by a high voltage outdoor switch yard. They introduced a new bio leach process which causes warm plumes of (they say HCl) but I think sulfuric acid or hydrogen sulfur. Anyways, in the space of only two months ALL the tinned copper overhead lines, copper busbars, silver-tin alloy connectors and such have turned jet black!
Reading the threads, it sounds like,a sulfur in air + humidity problem but we have no idea how the blackening may affect the reliability of the HV switchyard or how to treat the parts or if they are all replaced, how to coat the parts to prevent further problems.
Could anyone help me with this or even point me in the direction of the the kind of chemist expert we could use to investigate?
Kind regards, Tim
Metro Power Company - Perth, Western Australia
January 12, 2019
Q. We have yet not got rid of this problem of blackness on tin plating.
We are providing nickel undercoat, still the problem is persisting.
If any ne has found a solution and seen the results practically, please share asap
Thanks
Metalcast - Delhi, India
August 4, 2019
Q. I'm dealing with the exact same situation as Kyle. I have an old family Enterprise #22 cast iron meat grinder. It was my wife's great-grandfather's from North Dakota. She is 67 so that gives you an approx. age of the grinder. It is NOT rusted -- but IS completely black. It's in really good shape except for being black. There are some very small areas around the letters, and also some areas on the auger, that still have a faint tin-shine to them. I tried several different kinds of abrasive cleaners- and even a little bit of wire-brush in my drill- the only thing it did was make it shiny- but still black as night.
Question -- does ANYBODY out there know if it's safe to use at all, even if I clean it up really, REALLY well and then oil it up w/ food-grade mineral oil? Thanks -- Mark
tinkerer, overthinker - Montesano, Washington
November 7, 2021
A. Hi Mark, although no one can tell you that something of unknown provenance has never been exposed to anything toxic, we can tell you that it's very common for these tin plated cast iron meat grinders to be black after many years.
I'd look at it this way: if you've made sure it's clean, and you can't get any of the blackness to come off with detergent, or with several types of abrasive cleaners, and you've checked it with a white cloth or paper towel ... how is anything going to come off onto your food? Tin and iron are harmless in reasonable quantity anyway.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
![]() Ted--
Ted--
Thank you for answering! We've had it up in our attic for the last 4-5 year I guess, in my mother-in-laws house for maybe 20 years, and another 20 or way more in HER mother's- (wife's grandma's)- place. I'm gonna guess it was never exposed to anything toxic. I think it was just exposed to TIME. Probably lived most of its life in a basement or barn in somewhere in North Dakota. As I said- there is ZERO rust anywhere on it- just blackened tin I think. Think I'm gonna give it a REALLY good cleaning, seal it up w/ food-grade mineral oil, and get 'er grindin'!
Thanks again!- Mark
- Montesano, Washington, US
A. Try ammonium citrate solution (50 gms food grade citric acid ⇦ this on eBay or Amazon [affil links] , 1 lit water, add some ammonia ⇦ this on eBay or Amazon [affil links] until pH is 3,5 !). 2-10 % water solution of 47,5 gms citric acid ,47,5 gms sodium gluconate, 4,9 gms tartaric acid ⇦ this on eBay or Amazon [affil links] and 0,1 gms nonionic detergent can be helpful too...Hope it helps and good luck!
Goran Budija- Cerovski vrh Croatia
@Capacho Francisco How do you precipitate stannic ion? ... means how much phosphoric acid is used to precipitate the stannic ion?
Ashok Laxmanrao Gaikwad- Pune Maharashtra
July 28, 2022
⇦ Tip: Readers want to learn from Your Situation 🙂
many readers skip abstract questions.
Q. What if you have old terminals and just want to clean off the oxide off them?
Christopher Hoinskyhobbyist - Milford Connecticut usa
August 18, 2022
A. Hi Christopher. To do it right would depend on whether the terminals are bare copper, or tin plated, nickel plated, gold plated, silver plated (or something else); the current & voltage levels they need to sustain is important.
For casual applications (flashlights, toy trains, etc.) consumers tend to just remove the oxide with a mild abrasive, which will often work for a variable period depending on exposure conditions. Contacts which carry digital signals (usually gold plated) will not work unless the gold plating is restored. There are 101 ifs-ands-&-buts -- please try your best to describe your situation. Thanks.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. I am having issues with tin plating over 2-3 microns of copper on brass turning dark and zinc is then detectable in the tin. I am assuming this is de-zincification of the brass migrating through to the tin.
My question is, is it possible to measure copper thickness layer under the tin? So far I have had no luck with the Fischerscope. It can't measure the copper once tin is plated over it. From cross sections, it's where the cooper is thin that the tin goes black.
We can't use nickel strike as it's a telecom product and Nickel does strange things to RF performance and Passive Intermodulation.
So far we have seen it where 3 microns of copper has been measured by cross section, but not where the copper is above 5 microns.
Is there any way to measure tin-copper over brass? So far we I haven't found a way without cross sectioning
- Halifax UK
October 20, 2022
A. Hi Peter. I'm not an expert on the limits of XRF, but your report that you can't reliably measure a 2-3 µm thickness on brass under tin plating does not surprise me. Nor that the tin is turning black where the copper is thin, as you report. While 1 micron of copper can sometimes be enough, it may depend on the smoothness of the substrate and the general properties of the brass, and any acid exposure between copper strike and tin plating.
But it does seem to me that it may be more practical to simply apply more copper plating than to struggle with trying to hold it between 2-3 microns and then verify that it is. Similar suggestions run through thread 9676 and 1385 which are about tin plating on brass, and which emphasize the importance of a reliable copper plating layer.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread

