Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Who pickles stainless steel in the US?
Just curious who pickles stainless steel in the US. Is Fluoric/Nitric acid the most common system? And how do companies dispose of spent pickle liquor?
Thanks
industrialist - Dayton, OH
2007
Like many other things, the first battle you'll have to fight is semantics, Greg. Most electroplaters call their acid dip tank
(between the electrocleaner and the plating tank) a pickle tank, whereas steel mill operators would suggest that the platers should call their process an "acid activate". Similarly, operators of tank cleaning companies who travel to do on-site cleanup and refurbishment may call a step in their process 'pickling' as well. But heavy pickling is done at the mill and would usually involve, as you suggest, nitric-hydrofluoric acid. Disposal may be either via shipment to a recycler who reclaims the nickel and chrome, or by simple neutralization and filtration. Additional responses are encouraged.
Please give us some more info about why this interests you. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2007
2007
The traditional means of disposal would be neutralization with lime. This both raises the pH, precipitating nickel and chromium, and provides calcium ions to precipitate fluoride. Expect heavy sludge generation.
A recycling technology applicable to acid baths is acid retardation. The spenr bath is passed through a bed of an anionic exchange resin. Some of the acidity is retained in the resin bed via a phenomenon known as "site sharing."
The spent acid that passes through is now weaker than what went in, and will require less alkali to neutralize.
When the new bath is made up, plain water is passed through the resin, and some of the acid is recovered, without most of the metallic contaminants.
Rohm and Haas markets resins under the trade name Retardion that are specially adapted to this process. And, no, the other ion exchange resins don't make fun of them. :)

Dave Wichern
Consultant - The Bronx, New York
2007
David in correct that anionic purification works well for recycling and reusing spent acid.
|
|
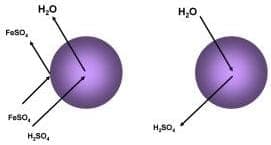
Eco-Tec developed an ion exchange system, called the Acid Purification Unit (APU), that specifically addresses this issue. It works with nickel salts, chromic acid, nitric, sulfuric, and hydrofloric acids at a rate ranging from 11 kg/hr to over 300 kg/hr (24 - 660 lbs/hr). Of course with recycling and reuse, other benefits can be obtained such as less sludge and reduced waste treatment costs. |
|
The retardation "site sharing" that David refers to can be seen in the drawing provided here: |
|
|
Lisa Hausz Eco-Tec Inc. Pickering, Ontario Canada |
|
Q, A, or Comment on THIS thread -or- Start a NEW Thread