
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Acid Copper Plating Problems Q&A
Q. I am having these rough patches in acid copper plating.


What can be the reason?
Ahmad Malik- new york [fictitious]
June 9, 2023
A. Hi Malik.
You've given us no info to go by yet, and unfortunately there are many possible causes for any given defect. But guessing from the photo and no data, maybe these components are zinc die castings full of porosity? If it is not a die casting, and you are sure the mechanical finishing was inspected before plating, my next guess would be floating grease being redeposited on the part as it's lifted out of the cleaning tank.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
⇩ Related postings, oldest first ⇩
Q. We are experiencing a problem with our acid copper. Our amps are dropping to zero while our volts are increasing and the plating has no shine. We tried different rectifiers and the problem remains. We are now carbon filtering the tank but it does not seem to be helping after 2 days. The tank is 200 gals. I'm at the end of my rope with too many deadlines unable to be met until the problem is rectified. Thanks...Don.
Don Prestageplating shop owner - USA
2007
|
A. If the anodes are phosphorized copper as they should be, then the anodes are polarized. Take them out, remove the bags, replace with new clean bags, put back in. The black sludge is "hazardous waste".  Robert H Probert Robert H Probert Technical Services Garner, North Carolina  A. Service anode or change to new. John Hu- Singapore |
A. Don,
The first thing you need to do is a complete analysis of your solution to check that your bath is in balance. Carbon treating without knowing what you are treating for is pointless.
If your analysis shows that the copper and acid levels are correct then do a Hull Cell and play with the brightener levels until you get a satisfactory plate in the mid range current densities.
If all else fails ask the chemical suppliers if they can help, they are usually extremely helpful and can normally offer the services of an expert to try to sort out your problems.
I must admit that it is a long time since I played with acid solutions, normally dealing with cyanide or pyrophosphate systems these days so I apologise for my vagueness but there are simple rules to apply to a situation like yours: Analyse, Hull Cell, expert, dump.
If your production pressures are high sometimes it is more economical to dump the solution and start again rather than have a long down time while you try to sort the problem.
Hope you get your problem sorted soon.
Aerospace - Yeovil, Somerset, UK
A. You will "lose" your ability to carry current when the amount of copper in solution has become too high raising your ratio of copper sulphate.
Titrate the solution and see if you are within spec; if not, the easiest solution is to cut the bath. You can test it out first in Hull Cells.
plating shop - Hbg, Pennsylvania
|
A. Hi Don plating - Turkey A. This is classic anode polarization and most likely due to too high copper sulphate ⇦ this on eBay or Amazon [affil links] content and possibly low temperature.  Geoffrey Whitelaw - Port Melbourne, Australia |
A. Hi Don,
The chloride level in your bath is low. The level must be above 40 ppm and below 80 ppm for satisfactory passage of current.
Do this and let us know.
Regards,

Asif Nurie [deceased] [deceased]
- New Delhi, India
With deep sadness we acknowledge the passing of Asif on Jan 24, 2016
A. Hi Don,
I believe your problem is TOO much chloride -- are the anodes getting a white coating on them? If so, take them out and scrub until you see clean copper.
You may have to do this a few times.
I had this same problem in my electroforming tank.
- Durban, South Africa
A. The total concentration of copper sulphate and sulfuric acid should not be higher than 300 g/l otherwise you get polarisation.

Sara Michaeli
Tel-Aviv-Yafo, Israel
A. Everyone has valid advice. Basically they have told you to check everything since you could have too much or too little. The most valid advice is to call your supplier. They generally will offer to run an analysis of your bath at no charge (this is included in what is called customer service) and since you are having a problem they should then offer their learned opinion of what the corrective measures are.
Before everyone jumps on and comments I would like to clarify that periodic analysis by the vendor is not a substitute for having the capacity to have routine analysis done on premises or locally, nor that every time you have a small problem go running to your vendor (enough people do to give me days when I wish cell phones had not been invented). But the vendor does have product specific knowledge to their formulations as well (usually) as having people with a wealth of knowledge. Also your supplier should be sure you have a Technical Data Sheet for the products and processes being purchased, analytical instruction and a trouble shooting guide.
One point I forgot, if you are using "city water" in your acid copper, you should have the necessary chloride without adding any. If you have been making large additions of water and not of acid or salts that would tend to raise the chloride level (maybe). Get an analysis and test panels done!
- Great Neck, New York
2007
A. I am sure that your problem is high copper sulphate if your bath solution about 100 liter only you must to get out 25 liter from your solution and put 25 liter ionized water good luck
Majed Janineh- Beith Lehem Israel
October 2, 2011
A. Dear Don,
At first I am not sure what is really the issue in your plating bath.
At first you need to analyze the bath very regularly specially if it's a 24-hr operation. Depletion of additives and other chemical varies in every panels loaded in the bath and that is why there should be a regular routine analysis.
1) firstly, how is the current density, is it calculated as per number of panels per rack.
2) how is the chloride content and how is the color of the anodes, black phosphorized film should be seen on the anodes.
3) what is your process, is it pattern or panel plating (for PCB process), should the color of the bath is greenish then it should be contaminated much with more organics on it. maybe there is a need to do carbon treatment and check TOC.
You need to investigate further and the assistance of the chemical supplier also will help a lot to troubleshoot the issue.
Thank you very much.
Regards,
Allan
- Singapore
November 11, 2012
A. Don,
your copper bath, no amps but voltage because copper anodes are polarized (if you see your anodes are dark color that means polarized)
pull out you anodes , change the new bags, new proper type of anodes, check your chemistry.
check the rectifier, check the current to anode and cathode bar.

Popatbhai B. Patel
electroplating consultant - Roseville, Michigan
September 25, 2018
A. Dear your copper sulphate is very excessive. Maintain copper sulphate 180 hm per ltr and sulphuric acid 30 ml per ltr
Your current dropping problem will be solved.
- Jaipur India
June 30, 2024
Reducing sulphate level in a Copper Bath
Q. Hi everyone, I am a student of Chemical Engineering in Brazil and recently I started an internship in a company that works with electroplating. We have collected cylinders for use in rotogravure.
I'm very new to the subject and it's been hard to find materials (handbooks are all sold in the USA and I can not find any PDFs), so I'd like to rely on your experience to answer the following question:
How do you make the correction of copper baths?
We work with 1000 L baths with H2SO4: 50-70 g / L and CuSO4: 200-220 g / L.
Here the baths are in contact with a very high amount of copper's chips, so with the passage of days the amount of sulphate increases a lot and to correct it the head of the sector uses the following strategy: Discard a certain amount of the bath (for example 100 L), complete the bath with water again and add more acid to compensate for what was lost.
In my opinion, we lose a lot of copper, acid and water every week. I've already suggested removing copper chips from the bath, but since I'm a newbie I do not have much credibility.
Another idea I thought was to precipitate sulphate with Barium Carbonate. I do not know if it works, but I intend to experiment with it.
Thank you for your attention and sorry by my English!
- João Pessoa, Paraíba, Brazil
December 3, 2017
A. Hi cousin Jerlan. I think your problem is more properly described as excess copper rather than excess sulphate per se; there is no place for sulphate to come from during operation, but dissolving anodes can contribute to excess copper.
You will read above that two responders do suggest your action of bailing out some of the solution and replacing it with water -- but I think this was suggested as an emergency fix, not as an operational routine.
The thing to investigate is whether you are using phosphorized anodes. These are intended to solve just this problem. After that is assured, then you might consider reducing the number of anodes and, if necessary, making a small and controlled chloride adjustment.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
![]() Thanks, Mr. Mooney! It really helped me.
Thanks, Mr. Mooney! It really helped me.
Sorry it took so long to answer :)
- João Pessoa, Paraíba, Brazil
December 28, 2017
Q. Good day. We are facing dots problem in copper plating; we did filtration properly but the dots are not reduced
gopi nath- Tamil Nadu, India
April 14, 2018
A. Hi Gopi. Please mail some pictures of these "dots" to mooney@finishing.com for posting here because it is not clear if they are roughness, pits, discolorations, or whatever. I assume they are pinhead size or smaller?
Durney's wonderful "Trouble in Your Tank" ⇦ this on
Amazon
[affil link] starts from the first paragraph with the reminder that the only way this can happen is because something changed, so your first step is to figure out what it was that changed :-)

Luck and Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Dear Sir,
I want to ask one question --
We are dissolving 230 g/liter copper sulphate and doing Hull Cell test, and it is coming glossy.
My question is if we are using copper sulphate 230/liter and doing Hull Cell test how should it come, and coverage of Hull Cell panel how should it come?
How we will know which Hull Cell is the best one -- dull Hull Cell panel which has 100% coverage or glossy one which has 90% coverage.
Please suggest.
Shiv Shakti Engineerings - Delhi, India
September 24, 2018
A. Hi Raj. Sorry, but your question is a bit too vague for me to be able to comment properly. Are you saying that you trying to do copper plating from 230 g/l copper sulphate and nothing else -- no sulfuric acid, no chloride, no addition agents?
Or are you implying that you are using a properly formulated bright acid copper bath, and gradually adding brightener, only to discover that as you move from dull to bright you start losing coverage? If so, can you tell us your chloride content? Brighteners and chloride work sort of symbiotically and sort of in opposition -- it can prove impossible to obtain proper plating while ignoring chloride level. Good luck and get back to us please.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Dear Sir
I am trying to do copper plating from 230 g/l copper sulphate and nothing else -- no sulfuric acid, no chloride, no addition agents.
I am doing hull cell testing in laboratory.
I am using 2 amp current for 10 Minutes
But Hull cell results are glossy and plating coverage is 90 percent
When I am doing hull cell from other company's copper sulphate, it is dull and Hull Cell coverage is 100 percent.
So which is best copper sulphate to use and why?
Shiv Shakti Engineerings - Delhi, India
by N. Kanani

on AbeBooks or
eBay or
Amazon
(affil links)
A. Sorry Raj, neither is satisfactory. As a wild guess the one which is offering complete dull coverage is purer and the other is contaminated with something which is brightening it.
You haven't told us what you want to plate and why, but the short answer is that you don't use plain copper sulphate -- that's for school science projects to demonstrate the science of copper plating, but not for actual industrial use.
Ideally you buy a bright acid copper plating process from a supplier, but if that is not practical for some reason, you formulate one from copper sulphate, sulfuric acid, a carefully controlled small amount of chloride, a wetting agent, thiourea ⇦ this on eBay or Amazon [affil links] , and a brightening agent like dextrin or molasses. If you can't get access to any books about copper plating, then the copper plating chapter of the Metal Finishing Guidebook is at least a good start. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Tip: Readers want to learn from Your Situation 🙂
(little can be learned from abstract questions, so many readers skip them)
Q. What is the best solution to reducing copper sulphate in copper bath?
Jessie Guerrero- Roscoe, Texas , USA
November 19, 2018
A. Hi cousin Jessie. Two readers suggest bailing out the tank as the most practical immediate fix, but the bigger question is how you got there. You have to fix that, and studying this thread should tell you how :-)
But get back to us with anything that's not clear. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. Hi Jessie,
Besides bath bail out, if your intention is to reduce Cu ion then you may use insoluble anode to run dummy plating till Cu drop to optimum level. If your intention is to reduce sulphate ion then you may replenish copper oxide instead of copper sulphate to minimize sulphate ion build up.
Regards,
David

David Shiu
- Singapore
Q. In bright acid copper plating bath we have an excess of foam. Can I use antifoam to reduce foam?
R MunirajuBangalore, Karnataka, India - Bangalore, Karnataka, India
December 25, 2018
|
A. Hi R Muniraju,  David Shiu - Singapore A. Hello, I assume you have air agitation, so some foaming is normal. Too much however can be caused by an excess of organics, commonly caused by brightener break down that occurs over time. Another cause would be drag in of a pre - clean chemical. Be certain that your rinse tanks are clean and your acid dip is also clean. One way to check possible contaminated rinses and acid dips is to grab a half liter sample in a bottle, shake well and check to see if sudsing lingers. If it is a brightener break down issue, carbon treatment is necessary. As far as using a defoamer, your current Cu chemical supplier can give you that answer. Personally, I don't know of any baths that have one. Hope this helps you. Mark BakerElectronics plating - Phoenix Arizona USA |
Q. I'm doing hull cell plating 1.5 A (using CuSO4 with sulfuric, Cl ion and brightener). I've got agitation mark at high current -- is it too much Amperage , or brightener excess or other causes?
molly charless- ipoh, perak, malaysia
September 12, 2019
A. Hi Molly. Please send a good photo of the panel to mooney@finishing.com for posting here. It's hard to photograph/see a mirror. If the copper plating is highly reflective, try to reflect a page of text on white paper onto your panel for the photo to help us see the distortion. Thanks.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
September 2019
Q. Hi,
I am plating antenna with copper sulphate and sulfuric acid (H2SO4). Which proportion will I mix them and which amperage to give it for 2 cm2 area of antenna. Can you help me please.
aybu - ANKARA,TUKEY
October 4, 2019
A. Hi Abdurrahim. If you are a hobbyist doing one antenna, try the following (from the Metal Finishing Guidebook 2012-2013):
199 g/L Copper Sulphate
30 g/L Acid
50-120 ppm Cl
If it's not bright enough to your taste add 0.0375 g/L thiourea ⇦ this on eBay or Amazon [affil links] and 0.75 g/L molasses. Current density about 1.5-3.0 A/dm2.
But if you are a plating shop doing professional work you should probably be buying your acid copper plating solution from a reputable supplier and following their technical data sheet rather than playing with generic formulations. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Bright acid copper... great results and grainy on the same piece
Q. Please see the attached pictures. I've been plating a piece and getting better and better results, but now I've gotten a bit stuck. I'm getting a lot of "bead" buildup in some portions, but pretty great results in others. I'm thinking it is due to the odd shape of the piece, but hopefully you've got some recommendations on something I've missed.


August 14, 2020
- Salt Lake City Utah
A. I have dealt with this problem before. We maintain three full time acid copper baths. In my personal opinion this looks like a chemistry issue. Try running a Hull Cell to see if your bath is lacking in brightener/carrier. I run mine at 1 ampere for 10 mins and if you have a lack in the brightener system components you will see a shaded or pitted area on the panel. Add small amounts of the corresponding component (I.E. brightener or carrier or both depending on the manufacturer of the solution you are using) to the Hull cell. I start off with small adds around .1mL at a time and run another panel. You should see an improvement in the deposit. Continue until you have a nice even panel with no irregularities in the finish. You can convert the adds you made to the hull cell to the main plating bath, amount will depend on volume of the main tank. One other thing you can check is solution agitation. Acid copper baths require a lot of agitation. Most Shops use air agitation or jet agitation through inductors. Air agitation tends to consume more of the brighteners than jet agitation. Something to keep in mind. Good luck, I hope this helps.
David Shade CEF CAF- Attleboro, Massachusetts
A. You are seeing the result of organic material in the acid copper bath. It could be accidental, from the mandrel or other source, or it could be "deliberate" from addition agent for grain refining or brightening.
Carbon treat the bath, run a sample. Then, if necessary, start adding your addition agent in very small quantities. Don't over-add.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
Q. Sir I am doing acid copper plating past 2 year my question is our components is almost 300 micron my question is when I am do plating first one hour I will give 250 amps but after one hour the amps will drop and volt will increase so please can you tell the corrective action.
Anandh kumar- India Tamil Nadu, Chennai
January 28, 2021
We'd like to give credit for this graphic explaining Ohm's Law (A = V / Ω) but see it on many websites without info of whose work it actually was :-(

A. Hi Anandh. Per Ohm's Law, A = V/R, so if the amperage has gone down when the voltage hasn't, the only explanation is that the resistance has increased, i.e., the conductivity has decreased.
There could be a number of possible causes, but without any other data, my first guess would be that your anodes become polarized over the course of the hour. Please search the site for "acid copper polarized anodes"
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Dear sir, I'm hoping you can help me with this issue we have been running in with our copper bath. Below are pictures of our problem and conditions we have been working with....







I hope you can give us some useful advice, Thanks in advance
Luka JelencicZagreb, Croatia
March 1, 2021
A. Hi Luka,
I've seen acid copper plated parts defect, due to over brightener use in acid copper bath. Run hull cell panel and adjust the chemistry, and carbon treat if necessary or dummy the copper bath.

Popatbhai B. Patel
electroplating consultant - Roseville, Michigan
July 10, 2021
? A lot of variations in process. Tank material and chemistry of bath?
Ijaz AhmadIncharge Surface Treatment Section - Islamabad
July 28, 2021
Ed. note: One of the graphics shows the chemistry of the bath and operating conditions.
A. It's a Acid copper plating bath in attacking
Solution ...
First use activated carbon packed in filter 1 gm/ltr, and running in tank minimum 4-6 hours.
And then dummy out at high current density.
Then bri.-0.01 ml/ltr part A
Part B - half of part A
Makeup - 0.1 ml/ ltr
Employee - New Delhi, India
September 25, 2021
Q. I am getting lots of tiny cubical mirror surface bright copper crystals formation from Acidic copper plating with 130 g/L CuSO4, 105 g/L H2SO4 and 80 ppm Chloride. Additives used are SPS + PEG8000 + 40%EO/PO + a proprietary leveler containing ammonia ⇦ this on eBay or Amazon [affil links] and nitrogen. I have tried extensive carbon treatment to restart the bath without success.
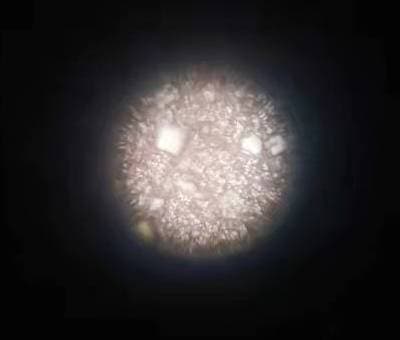
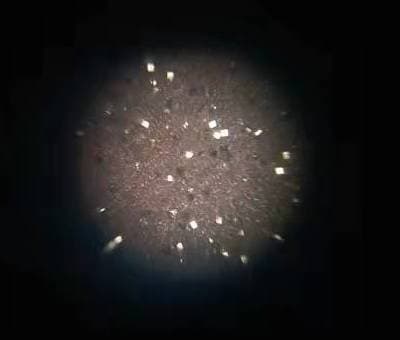
Has anybody ever encountered this type of shinny reflective crystal spots grown along on copper surface before and may suggest or share a solution to eliminate them? They are considered defects for our product finishing.
Many thanks for your time and helps consideration...
Electroplating Manager - Hong Kong
January 10, 2022
? Hello Andy, I have a few questions for you before I give you any troubleshooting tips and remedies. When you run your hand over the copper plated surface, is it smooth or rough? Are your anodes bagged and if so, are they in good shape? There shouldn't be any holes in the bags.
The reason I ask if the surface is rough where you're seeing the crystals is that there is a very good chance the problem would be particulate matter in the bath. You will also want to make sure your filtration is adequate, with filters that are changed on a regular basis. Please let me know the answer to my first three questions. Thank You and good luck.
Retired - WINSTON SALEM, NC
Q. Hello Mark,
Thank you very much for your response.
1. Copper plated surface is like a very fine sandpaper and so I would say it is rough.
2. Anode bags are good as I tried even washing & cleaning all my anodes with new bags enclosure. The bright spots remain.
3. Filtration should be adequate as I tried changing new filters as well but no help to the problems.
My current suspicion is that there might be problems existing between SPS and MPS redox reactions. During plating with PEG suppressor, SPS breaks down to MPS who is the actual accelerator for copper reduction, somehow these MPS are not able to oxide themselves back to SPS forming an unbalance redox reaction favoring MPS to exist which then might has caused the formation of these bright regular cubical facet crystals with mirror reflective surface.
So I think I might have overdosed my plating bath with PEG and EO/PO (an nonionic wetter) as they may now be suitable to co-exist with bath.
Expert advice would be appreciated on below questions:
1. Is it wrong to mix PEG 8000 (ionic or not?) with EO/PO (an nonionic wetter) in plating bath together?
2. Would the above mixing of PEG and EO/PO cause redox reaction between SPS and MPS to become outrageous?
Many thanks in advance.
Very Best Regards,
Andy
- Hong Kong
A. Hello again Andy,
I have to be honest with you in that I have never heard of the additives you mention for an acid copper bath. I guess I've been out of the business too long. I have worked for companies that had to comply with many mil specs and certifications, but I'm afraid I can't help you with this.
Retired - WINSTON SALEM
Q, A, or Comment on THIS thread -or- Start a NEW Thread
