
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Electroplating with Brass (Electrodeposition of Brass)
Quickstart (no reader left behind):
Brass is an alloy of two metals, copper and zinc. It would seem impossible to electrodeposit brass because copper is so much more "noble" than zinc, "wanting" to deposit while the zinc "wants" to stay dissolved in the solution. This difference in potential is more than a volt, so strong that school children make batteries by putting a piece of copper and a piece of zinc into a mild acid, where the copper simultaneously drives the zinc into solution and coats it until no zinc metal is exposed to the acid.
The only way to electrodeposit brass is to keep the concentration of zinc ions in the solution millions of times higher than the concentration of copper ions. This is accomplished by using a complexer like cyanide which releases copper ions so very sparingly that zinc ions outnumber them by millions to one.
Tip: Readers want to learn from Your Situation 🙂
(little can be learned from abstract questions, so many readers skip them)
Q. Is it possible to electroform ⇦ learn moreElectroforming is the same thing as electroplating but is called "electroforming" when the plating becomes the object rather than a coating on the object. Hollow gold earrings, for example, are electroformed by plating onto wax, then dissolving away the wax leaving just the gold electroform. brass on an object. Same as like copper electroforming?
Please expert suggest and reply.
- Gujarat vadodara india
November 27, 2023
A. Hi Ankit,
There is actually very little difference between electroplating and electroforming except that electroforming is often thicker. So, if you need only a very thin electroform layer, yes, it can be done in brass.
However, brass is an alloy, which means it needs strong complexors, whether cyanide or proprietary, and the more you ask of it, the more it can go wrong. Even "heavy brass plating" is only about half a mil in thickness (0.0005"), and I would probably expect trouble at greater thicknesses. Is there a reason you can't electroform whatever it is out of copper and then apply a decorative brass electroplate onto the copper?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
⇩ Related postings, oldest first ⇩
Q. Sir,
We currently do Copper plating & then brass plating on M.S. Hinges. I would like to know
1) is there any problem in doing brass plating over cyanide copper plating on M.S. articles
2) Is there any risk in doing only brass plating on M.S articles, even if the lacquer is of good quality?
Raj Industries - Delhi, India
2002
A. The first approach is fine; I'm not sure that there is a theoretical reason you cannot plate brass on steel, but platers almost universally plate it with copper first. If you are looking for bright brass a better way to go may be to start with with bright nickel plating, rather than copper plating, under the brass.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Brass is an alloy of zinc and copper. How then is it possible to electroplate with brass? Why doesn't the brass dissociate in the bath into copper and zinc?
Gordon James Stoppshobbyist - Toronto, Ontario, Canada
2004
A. Hi Gordon. We've now added a "Quickstart" to the top of the page. Brass does dissociate as copper and zinc ions, and the ions are reduced to copper and zinc metal, creating the brass alloy.
Yes, copper is much much more noble than zinc, so we would expect the deposit to be 99.999% pure copper if it were plated from a simple salt.
But when copper cyanide, zinc cyanide, and free cyanide are mixed in a proper ratio, the cyanide fights very hard to hold onto the copper, so the concentration of free copper ions is extremely low, so you are able to plate the brass alloy. Cyanide is a dangerous chemical and there are other complexers these days that allow brass plating -- but you'll never plate brass out of a simple acid like chloride or sulphate.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Need to Speed Up Deposition in Brass Plating
Tip: Readers want to learn from Your Situation 🙂
(little can be learned from abstract questions, so many readers skip them)
Home Hardware - Caxias do Sul, RS, Brasil
2003
A. Hi Diogo.
The only thing that can and must be done to increase plating speed is to increase the current density -- because moving electrons from the anode to the cathode via electric current is, according to Faraday's Law, what makes a proportional number of metal ions move from the anode to the cathode.
by Abner Brenner

on AbeBooks
or eBay or
Amazon
(affil links)
How to achieve this, while avoiding consequent problems like burning, is, of course, difficult to resolve in the abstract. But impelling jets of solution onto the cathode at high speed is perhaps the most promising way, since it minimizes the thickness of the boundary layer through which the ions must diffuse and, by rapid replacement of solution, it also keeps plenty of metal ions available, rather than having the area starved for metal.
Are you using a cyanide or non-cyanide brass plating bath, Diogo?
It may be possible to increase the temperature of the bath, which leads to greater ion mobility and thus theoretically allows faster plating speed, or to increase the metal concentration -- but these things may also affect the "balance" of the bath (Copper is far more noble than zinc, so it's quite a trick that we are able to plate brass at all :-)
You might be interested in Brenner's "Electrodeposition of Alloys", because as you change voltages, currents, temperature, and concentration you have a propensity to change the alloy composition.
Best of luck, and get back to us.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Good evening,
I need the answer for the following questions in detail:
Q1: when we use low voltage in the electroplating of brass, we noticed that the zinc ions are deposited rather than the copper and the color was white? We use brass plate as anode and spoon as cathode and brass solution contains:
- 40 grams of brass salts (copper sodium cyanide and zinc sodium cyanide),
- sodium sulphite, and sodium cyanide,
- and we add ammonium chloride to the solution
We fix the temperature, distance between cathode and anode, pH (9.8-10.8) copper content 60% and zinc 30% in solution and just change the voltage.
Q2: Write the oxidation-reduction reaction which occurs in the brass solution?
Q3: Why do we use ammonium chloride and sodium sulphite and sodium cyanide in this solution?
Q4: What are the advantage of using the cyanide bath in brass electroplating?
Brass solution : Zonax brass 75 g/l and 3 g ammonium chloride. Brass contains 70% copper And 30% zinc
electroplating student - jordan-amman
April 15, 2014
A. Hi Ammar. Sorry that no one helped you yet. Perhaps if you start with one particular question where you need help, the results will be better; no stranger is likely to provide your entire lab project report for you :-)
But for Q1: As mentioned earlier, copper is far more noble than zinc, and will preferentially deposit from a simple salt. To counteract that and make brass plating possible, it is necessary to complex the copper with cyanide, to very greatly reduce the number of free copper ions available for electrodeposition. Even when this is done, however, it requires careful balancing of all parameters to get a balanced deposit of copper and zinc. Your experiment demonstrates that if the voltage is very low, things go out of balance, with the copper too complexed for any of it to deposit; but if you increase the voltage, you overcome the complexing sufficiently to deposit a proper proportion of copper and zinc.
It is noted in Lowenheim's "Modern Electroplating" →
that with some brass plating baths the proportion of zinc rises with higher current density, and for others it decreases. He includes a graph by Compton, Ehrhardt, & Bittrich for one bath which exhibits highest zinc concentration at about 0.3 A/dm2, and declining whether the current is increased or decreased. Lowenheim has an excellent theoretical and practical treatment of brass and other alloy plating baths. Please try to find a copy in your school library; if it's not there, and you can't find another good book on the subject, please suggest to your advisor that Lowenheim's book be obtained. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Sir,
Please explain to me about Brass electrolyte.
Rate of deposition of zinc and copper?
What is the ratio of metal deposits from electrolytic solution and anode?
- Delhi, India
April 4, 2016
A. Hi Santosh. Most brass plating electrolytes are cyanide-based, and the cyanide complex allows practical ratios of copper and zinc to be deposited for brass deposits (today there are also some proprietary brass plating baths available that do not require the use of cyanide).
"Modern Electroplating" describes the composition of 10 different cyanide-based plating baths and includes 233 references. It is the best starting point that I know of for your theoretical investigation; but what some readers visiting this page may not necessarily realize is that, in general, users do not attempt to formulate electroplating baths like you see in school; rather they purchase them from suppliers who have spent years or decades perfecting them, and offer them as proprietary processes. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. The Cu/Zn ratio in the deposit is not, as you might think, determined by the Cu/Zn ratio in the bath. It depends more on the cyanide/zinc ratio.
pH control is also crucial. There are two different levels at which brass is plated - one in the mid tens, one in the upper 11's, if memory serves.
Do some library research. This information is out there.

Dave Wichern
Consultant - The Bronx, New York
Q. Want to do natural brass finish plating (reddish brass finish) on nickel plated or copper plated surface; we are using cyanide based bath.
A. Vasanth karunakaranEmployee - Chennai, India
February 28, 2019
A. Hi Vasanth. Red and other color patinas can be applied to brass, but I think I am understanding you to be saying you just want your deposited brass plating to be more on the reddish side, i.e., higher in copper. The previously mentioned "Modern Electroplating" [on AbeBooks, eBay, or Amazon affil links] has all kinds of graphs on the subject, and notes & exceptions & provisos, but the general trends are that higher current density, higher temperature, and lower pH generally move towards higher copper content. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. My company has met some problems of electroplating with brass (imitation gold color). We are using cyanide-based bath and our problem is that when we finish the brass plating after water cleaning, blowing dry and baking, there would appear not obvious red stripes on the sample surface. At first, we think that was caused by the water stain but after we strengthen the blowing dry the red parts remain. Can anyone give some suggestions to improve our problems.
Jimmy, Chungziyong enterprise co., ltd - Tainan, Taiwan
April 25, 2019
Q. Hello sir, I am doing a lot of work for developing cyanide based brass salts as follows: 70% copper cyanide, 35% zinc cyanide, ammonium chloride, sodium cyanide as free cyanide, sodium bicarbonate, sodium sulphite and potassium sodium tartrate ⇦ this on eBay or Amazon [affil links] and a bit of sodium thiosulphate ⇦ this on eBay or Amazon [affil links] ... but I did not find the satisfactory result. Please suggest what should I do to obtain yellow colour of brass ... please help.
Mohd malik- Tamil Nadu, India
October 12, 2020
A. Hi Mohd. There are many possible formulations for brass plating. Yours may be a perfect one, but unless a reader has worked with that particular formula they may not be able to suggest what needs adjustment. So please tell us where you found it because with a reference people can understand better.
Here's what Lowenheim suggests in "Electroplating" ⇦[this on eBay , Amazon, AbeBooks affil links] :
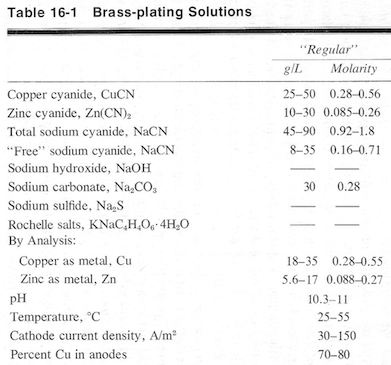
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Thank you so much for your valuable reply.
Sir I am working with electroplating industry since last seven years and already developed rochelle copper salt, chrome salts, nickel sulphate and chloride and copper sulphate. Sir I had developed these products by doing a lot of study by many means like some patents, etc.
Please please suggest me what should I have to do for brass salt if I want to make it only 10 kgs. What will be the compositions for this salt ... what should I have to add in it or remove from it. Please help me sir.
- Tamil Nadu, India
October 13, 2020
A. Hi again, Mohd. I think you misunderstood me :-(
I am not a chemist and have not spent years developing formulations like you have. My point was that we can offer you published formulations for brass plating solutions, but I don't have personal ones to offer you; and I doubt that anyone can simply look at your formulation and tell you what needs tweaking :-)
Here are a number of formulations from Lowenheim's "Modern Electroplating" [on AbeBooks, eBay, or Amazon affil links] :
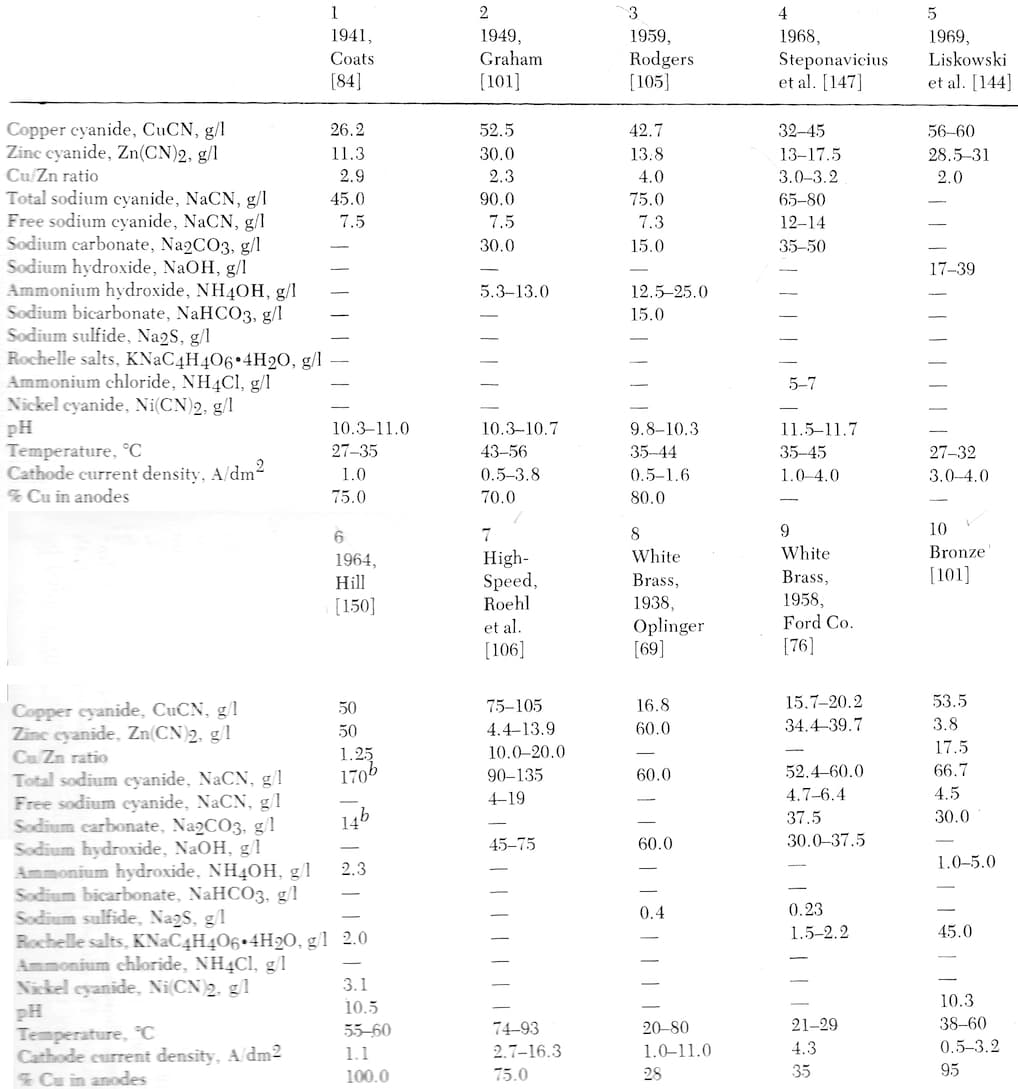
|
Note to readers: We generally don't copy charts or tables from books, potentially depriving authors of the reward for their efforts. But we made an exception with these two Lowenheim books because: he passed away 40 years ago; he was a luminary whose work is not recognized widely enough; his daughter Trudy Hayden found us, and gave us for free a bunch of copies of his books, encouraging us to distribute them. His family is not losing any money via this entry, and in fact they are very happy to see us try to help his work become more widely used and appreciated. |
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
|
|
- Orillia, Ontario October 15, 2020
Good luck with the brass railing, I think you'll find brass-tone if not real brass plated railing widely available. Luck & Regards,  Ted Mooney, P.E. RET Striving to live Aloha finishing.com - Pine Beach, New Jersey |
Brass plating iron - What solution is required?
Q. Hi there,
Greetings,
I want to plate iron using brass (Yellow copper). I have good information about electroplating but my question is how to make the standard bath solution with minimum chemicals because nothing is available in my country.
Thank you and appreciate your help.
Regards,
- Sulaymaniyah KRI Iraq
October 26, 2020
A. Hi Awder. We appended your question to a thread which includes the brass plating formulas from Lowenheim's books. But you should probably check the libraries for a copy of one of his books because there's more to surgery than selecting the scalpel :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Hi,
I'm working on setting up non-cyanide bath for brass electroplating in my shop. Can you please give me what chemicals I need to prepare the solution?
I found below from a source:
Copper sulphate 5-10 g/L
Zinc oxide 0.3-1 g/L
Sodium hydroxide 60-100 g/L
Glycolic acid 5-20 ml/L
Operating Conditions
Range
Current density 10-12 ASF
Additives Gelatin at a concentration of 0.5 g/L recommended for a smoother deposit.
Can I go with that?
I read many books for the past 2 months but still I need your consultation.
Thank you so much.
- Iraq Baghdad Center
November 6, 2020
A. Hi Ahmed. What is the source of that formula? We don't like to post formulas except with their published sources because we are concerned about violation of copyrights and crowd-sourcing of trade secrets.
I hope someone can help you, but Lowenheim says in Modern Electroplating (1974):
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Thank you Mr. Mooney for your reply,
Actually the source of the above formula returns to one of my chemistry friends and the copyright is well noted.
But I considered more than 5 books and sources that there is a way to accomplish non-cyanide bath plating at least in a small scale.
I hope someone could help me on this regard.
Appreciated
- Baghdad Center, Iraq
A. According to old Russian book, F.K.Androshenko, V.V.Orehova, K.K. Pavlovskaya: "Pirofosfatnye Elektroliti", Kiev 1965. you can try next 2 solutions:
1.
CuSO4 ....1-2 gms
ZnSO4.....1 gm
Na4P2O7...60 gms
H3C2O4----10 gms
Na2CO3....30 gms
Water......1 lit ,1A/dm2,18-20 °C temp.,brass anodes(min 80 % Cu)
2.
CuSO4 ....1-2 gms
ZnSO4.....0,8-1,5 gm
Na4P2O7...50-60 gms
Na2CO3....10-15 gms
H3BO3.....4-8 gms
Water.......1 lit,0,2-0,3 A/dm2,18-20 °C temp.,brass anodes(min 80% Cu)
Water......1 lit
Hope it helps and good luck!
- Zagreb; Croatia
Expired US patent US 2011/0052937 A1, pyrophosphate brass plating.
Hope it helps and good luck!
- Zagreb, Croatia
Q. Hello,
I still don't find a bright yellow brass plating using all the formulas suggested by you. Please help.
- Tamil Nadu
March 8, 2021
A. Hi Mohd. If you've tried all 14 formulations offered on his page, you can search the site for other threads like topic 4076, "Brass Plating Is Too Yellow" if your brass is not yellow enough.
But maybe the problem is no more complicated than not realizing that you should have bright nickel plating under the brass? Please get back to us about the bright nickel plating under your brass plating or the lack of it. The usual procedure when you want dull brass is to plate directly onto the steel, but when you want bright brass to plate onto bright nickel plating.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Hello sir
As we had some earlier conversation over brass plating ... after buying so many books I still don't get yellow brass colour ... please help.
- Tamil Nadu [returning]
October 10, 2022
A. Hi Mohd.
Apologies. As previously noted, I haven't done hands-on brass plating formulation, and can only offer you book knowledge. But we've given you over a dozen formulation tweaks from the industry's luminaries, and referred you to topic 4076 where everyone's brass is "too yellow!". If your brass is not yellow enough then there would seem to be enough info on that "too yellow" page to solve the problem. Good luck!
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Tip: Readers want to learn from Your Situation 🙂
(little can be learned from abstract questions, so many readers skip them)
Q. The process for the electrodeposition of brass.
Desmond Monday- Lokoja kogi Nigeria
March 23, 2021
A. Hi Lokoja. We appended your question to a thread offering over a dozen formulations. Please take two or three paragraphs to fully describe your situation. Thanks.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Hi, I need help with ternary alloy codeposition. I'm not from materials background, so any help is appreciated.
1. I need help with the electrodeposition test set up. Is it correct to have:
- the metal ions baths mixed together in a glass container
-potentiostat
-cathode, Pt electrode as counter electrode, Ag/AgCl electrode
-NaOH to make it alkaline solution, tartrate solution.
-temperature controller?
I've read several papers that give me different kinds of set up and I'm kind of confused. Is this kind of set up acceptable? Let me know if I miss anything.
2. Is it possible to measure the mass of each metal deposited to cathode during electrodeposition is performed?
3. Is it possible to measure the thickness of deposition during electrodeposition?
Thank you!
Student - KL [Malaysia]
January 3, 2022
A. Hi Nurul. I understand that you want to deposit a ternary alloy but an important question is why, i.e., what is your actual project or assignment? Because 99% of what we can tell will probably be worthless information to you. For example, and for starters, by far the best way to deposit a ternary alloy is to buy a proprietary plating solution already developed for that purpose...
The reason is that the three metals will have greatly differing deposition potentials; so, if you just mix ions of the three different metals together, copper will deposit to the virtual complete exclusion of the other two metals even if you put 1000X as much of them into the solution. The only way you will get an alloy that isn't almost totally copper is by tightly complexing the copper with cyanide or another efficient complexer. Are you trained and equipped to deal with cyanide?
But maybe buying a proprietary plating solution totally defeats the purpose of your project? So please take two or three paragraphs to explain who you are and exactly what your project is that requires you to deposit a ternary alloy. Any particular alloy? When we fully understand exactly what you are trying to do and why, I'm pretty confident that we can expeditiously help you.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q, A, or Comment on THIS thread -or- Start a NEW Thread

