
-----
What Metals Rust the Fastest (Steel, Copper, Bronze)
Q. Hi
I have to do a science experiment, "Which metal corrodes the fastest in water"
Do you have any tips on how I could do this? Or which metals I could use? Any tips/ information will be greatly appreciated.
I am going to test them in tap water in an indoor shaded environment, in containers of equal sizes and capacity of water. I don't think it is feasible to make this large amount of distilled water and purchasing it in my area is quite costly (unfortunately).
Lastly: should I use plates or wires? I think I should use wires but please tell me the better one.
Thank you :)
- Sydney, NSW, Australia
February 19, 2013
Q. Hello,
I am going to be doing a 10th grade chemistry experiment with a partner that has to do along the lines with:
-What metal rusts easily
-What can easily remove the rust and the amount of metal composition that has been removed during the rust removing process
-What solution/chemical composition has retarded/sped up the renewal of rust on the metal
I know what exactly my partner and I are going to use and what we to do but the question is what metal do we use to easily demonstrate the easiness/hardness of removing rusts with different chemical compositions/solutions. Do you have any idea that help us bring our science project "to life" as you may. Any ideas will be very helpful in this.
- North Hollywood, California, United States
April 21, 2013
A. Hi. I think you bring a project to life by picking something that already fascinates you. If you pick something that hasn't grabbed your interest by 10th grade (metal rusting ... ho hum), it probably won't now; and if you're not interested, it's hard to interest others. Try to think of your past or present hobbies, interests, models, etc., and how rust might have surprised, interested, confused, or inconvenienced you. Maybe your interest was bottle caps, model boats, electric trains, old cars, yard art, antiques, old computers, political buttons, musical instruments, magic tricks, or whatever. You probably won't convincingly feign enthusiasm. Think hard about what already interests you and how rust might impact it. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
April 22, 2013
Q. I have the same project. But I will test steel and iron but what other metal should I use?
Grant M [surname deleted for privacy by Editor]- Danielsville, Georgia, U.S.
October 3, 2013
Q. How long does it take for iron or steel to rust?
Bobby T [surname deleted for privacy by Editor]- Washington, DC USA
November 9, 2013
A. Hi Bobby. The Iron Bridge crossing the River Severn in Shropshire, England has been standing since 1781. But steel and iron can flash rust in 15 minutes -- so it depends on a lot of things.
But if you are asking how long it will take a plain iron or steel nail to rust when immersed in water for a science project, I think you'll see rust in one to two days. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
November 11, 2013
Q. I've read this entire thread and really appreciate all the information. For my son's science project he tested how long it takes for a bronze coated steel fishhook to rust. The liquids he used were saltwater, fresh water, coke, gatorade, and apple juice. Only the salt & fresh water hooks rusted; the others all showed signs of corrosion (black spots on the bronze finish). I'm trying to help him determine what would cause the salt and fresh water hooks to rust since we know (after reading the posts) that bronze does not rust. Why would the bronze finish break-down allowing the steel hook to rust? Would greatly appreciate some insight. Thanks!
Malynda Vassallo- Diamondhead, Mississippi, USA
January 10, 2014
A. Hi Malyda. While wearing goggles, snip or sand the barb off those hooks before using them in a kid's science project -- you're making me queasy :-) Although the ancients used bronze fish hooks, you are correct that today's "bronze" hooks are made of steel and are merely the color of bronze. I suspect that bronze hooks are not electroplated with bronze, but simply dipped in a bronze colored lacquer, but would appreciate if someone who works at a fishing tackle company would weigh in on this.
The sentiment on a number of fishing forums is that bronze hooks are the cheapest and least durable of any common finish. A Maryland DNR study found that no fish hooks dissolve away in a practical time period, but that bronze lacquer hooks may be the safest for fish, but probably not by a statistically provable margin (articles.baltimoresun.com/1991-03-20/sports/1991079022_1_barbless-hooks-hook-styles-smaller-fish)
This bronze lacquer is apparently not very impermeable, and does permit the underlying steel hook to pretty rapidly rust, so that's why they are rusting.
Coke, Gatorade, and Apple Juice are all mild acids (www.21stcenturydental.com/smith/education/pH_drinks.html). Rust is more soluble in acids than steel is, with the result that things may not look rusty even if they are corroding faster than they are corroding in water because the rust is dissolved and not visible. When the Coke, Gatorade, and Apple Juice eventually evaporate away, you should see significant rust in the residue.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
January 2014
Q. Hi my name is Bianca and I am a little flummoxed as to where to start on my 15 year old nephew's science project. We need all the help we can get because it is detrimental for his school certificate. It's about testing a factor that may affect the reaction rate of a metal/acid reaction. Which would be easier to choose and how do I begin?
1. Concentration of the acid: more concentrated acids increase the reaction rate.
2. Surface area of the metal: a larger surface area increases reaction rate.
3. The type of acid: more active acids increase the reaction rate.
4. The type of metal: more active metals increase the reaction rate.
PLEASE HELP.
- Sydney, NSW, Australia
June 5, 2014
A. Hi Bianca. Unlike your nephew and his fellow students, you haven't been attending science class and covering the subject in school (and receiving those little hints from the teacher), so I would expect you to be having a lot more difficulty with it than them :-)
First things first, are you sure he 100% understands the question? 100%. It's fine to ask a question if don't know the answer, but it only adds still more confusion for him to ask for an answer to a question that he doesn't understand.
Assuming he's past that hurdle, it looks like he's been offered the option to demonstrate any of four factors that affect the reaction rate. I can easily think of two more factors: temperature and agitation. So after he picks one, he has to make sure he can hold the other factors constant, because if they vary they may screw up the cause-and-effect results.
Do any of the 4 factors interest him? Interest is key to a good project! Most kids are more interested in "3." & "4." than the others. But "3." can be very hard to do in some of today's chemophobic classrooms, where real chemicals are often forbidden and the children are expected to learn chemistry from playing with secret recipes like Gatorade & Dr. Pepper (how do you know the acid is responsible for differences when you don't even know what chemicals are in secret recipes? -- it's ridiculous). So "4" is perhaps the project you'd better do for/with him. You can read on this page some of the great approaches to doing "3." or "4.", or combining them, by kids his age and even younger. Also, make sure you have his Science book in hand when you do this, because if you express anything that contradicts the language of his textbook there will probably be demerits. Good luck!
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
June 2014
Q. What metal corrodes the fastest? I am doing an experiment on how fast can a nail corrode in 3 different substances. They are water, salt water, and hydrogen peroxide. What kind of nail should I use?
sophia e [surname deleted for privacy by Editor]- little rock arkansas USA
September 25, 2014
A. Hi Sophia. Try to find plain "cut" masonry nails if you can. These are hardened steel with no corrosion proofing. But if you don't readily find them, then just "bright finish" nails.
What you need to avoid is "roofing nails" or "hot dip galvanized" nails as these are as corrosion proof as practical; and you also don't want stainless steel, or aluminum, or painted "paneling" nails.
But be careful in describing your project! Although you want a metal that corrodes fast in order to speed the project along, your subject is NOT what metal corrodes the fastest; your project is which of those three materials corrodes steel nails fastest. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
September 2014
Q. I'm 12. I was wondering if rust can be made from different types of iron, steel, etc. If so, how?
ella j [surname deleted for privacy by Editor]- george town,tasmania
October 21, 2014
A. Hi Ella. There is a lot of info on this page already. Simply putting a steel or iron nail, or steel wool ⇦ on eBay or Amazon [affil link] into water, salt water, or bleach will make it start to rust within a couple of days.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
October 2014
October 23, 2014
The cut nails are by far the best ones to use, but while there is no intentional preservative on them, most are heavily oxidized. Make them shiny with steel wool.
Bright nails normally have a thin coat of zinc electroplated on them, Here again, make them bright steel by a lot of rubbing with steel wool. If you want to make sure that you have removed all of it, put a sample nail in vinegar
⇦in bulk on
eBay
or
Amazon [affil link] will corrode metals but will dissolve rust faster than metal, confusing the issue.
The precious metals (gold, platinum, etc.) will not ever corrode. Copper, brass, zinc, nickel, tin and aluminum will corrode, but not rust.
But I don't think you'll want an experiment with multiple variables like all kinds of metals, all kinds of corrosion conditions, and all kinds of preservation techniques. I think it will be better to pick one metal (maybe steel nails), and one exposure condition (salt water OR fresh water, spritzing OR full immersion) if you intend to try multiple corrosion-proofing methods.
Corrosion-proofing methods could include a variety of store-bought paints, vs. dipping in grease, dipping in melted chocolate, waxing, etc.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
November 2014
Q. As a follow up to my last post, I want to rust the metals using full immersion. A major reason being it will rust the metals to a greater degree and quicker than spritzing. However, a major disadvantage to full immersion is that it limits the methods of prevention that can be used, as methods like a protective paste or coating will not still be adhesive after immersed in water. With only a experiment time span of around November to late January, will spritzing cause rust of a severe enough degree that there will be a difference when a prevention technique is applied? I have to review this problem and the problem with limited prevention methods with full immersion with my science teacher. Also, because metals like copper corrode rather than rust, do you consider rusting and corroding similar enough to be rated on the same type of scale? Does rusting and corroding happen for the same reasons/causes? Also, as you mentioned I stay closer to one variable for each topic, I plan to stay with one kind of corrosion condition, but my teacher will not allow me to stay with one metal; she believes I must expand by testing several metals with several methods. Your suggestions for simple corrosion-proofing techniques seem interesting and possibly applicable!
Matthew [returning]- Westerly, Rhode Island, U.S.A.
November 2, 2014
Q. I am a 10 year old. I want to know which metal will rust fastest (zinc, iron, or aluminum)? I need each one in salt water or tap water.
isaiyah g [surname deleted for privacy by Editor]- Wilmington, North Carolina,USA
November 17, 2014
A. Hi Isaiyah. Please have a parent read and interpret this page for you because it has been explained many times on this page that zinc and aluminum cannot rust (although they can corrode). Yes, you need each one in salt water or tap water -- so start a lab book, writing down everything you do and observe. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
November 2014
Q. Which Corrodes faster -- steel of higher grade or lower grade? And why?
Dayo Osa- Odessa, Texas, US
December 29, 2014
A. Hi Osa. If by "higher grade" you mean a more expensive alloy steel with significants amounts of nickel, chrome, or other alloying materials in it, the higher grade steel will corrode slower. A basic reason is that these other metals are not as corrosion-prone as iron, and they form tighter and more adherent corrosion products. 18% chrome essentially makes type 400 stainless steel; 18% chrome and 8% nickel makes type 304 stainless steel.
Even a small amount of alloying metal, like 2% copper or 3.5% nickel can significantly reduce corrosion.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
December 2014
|
Q. Thanks Ted. - Odessa, Texas, US December 30, 2014 A. Hi again Dayo. You do realize that you have now asked an entirely different question, I hope? Corrosion allowance is a minor factor in a pipe design, and the ultimate strength of steels is not closely related to their alloy content. There are many issues involved in material selection besides ultimate strength, which is why you see so much copper piping and plastic piping. And you need to be very careful if trying to equate maximum allowable pressure with ultimate strength because materials are generally sized based on their yield strength rather than their ultimate strength. Further, the strongest and hardest materials may be unsuitable for pipe, both because of the difficulty of fabricating such materials (how do you cut the threads if the pipe is as hard as a file?) and the fact that you don't want pipes made of brittle materials (consider files and drills; they're very strong and hard, but subject to cracking like glass instead of bending). A pipe failing with a slightly split seam after decades of use is still a problem, but a pipe made of brittle hardened steel bursting like a hand grenade would be a catastrophe. Please explain your situation. There's a big difference between a student question about a general theory, and practical design issues. Thanks. Regards,  Ted Mooney, P.E. Striving to live Aloha finishing.com - Pine Beach, New Jersey Ted can be retained for immediate answers or long term project help December 2014 |
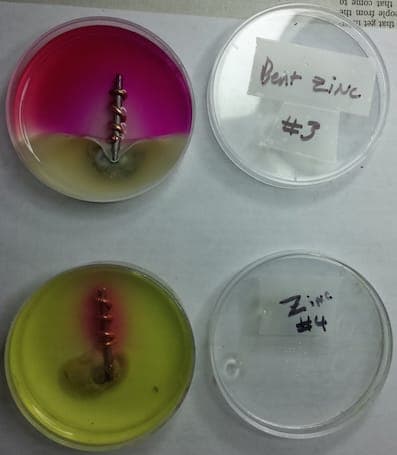
Q. For my son's 9th grade science fair project, he put samples of dissimilar metals in a corrosive environment, iron and copper. For some he added a zinc as a sacrificial anode (penny with the copper removed). On a lark, I suggested taking some of the pennies and bending them back and forth a bit before attaching them. We found the bent pennies corroded a lot quicker than the non-bent pennies, though we aren't sure why. Does the bending create more surface area? Does it affect the crystalline structure, which impacts the corrosion? Can you suggest an experimental way to figure this out?
Thanks! Scott
- Alexandria, Virginia USA
January 3, 2015
Hi Scott. Putting metal into tension does affect the structure and cause galvanic hot spots. The bending also might have increased the surface area. Unfortunately I don't know a quick and easy way to tell which was the more dominant factor. It might be possible to anneal the zinc to remove the stresses and see what happens then.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I am 20 years old and working on a project for my architectural studio class and I am looking up different materials to use on our structure. Looking into different metals, I was wondering what were some of the pros and cons to using Steel, copper, and/or bronze -- taking weather and aging into account. The part that the metal would be used for would be ribs for a bike rack on the outside of the structure.
nathan adams- Lincoln, Nebraska, USA
February 27, 2015
A. Hi Nathan. Steel will quickly rust unless it receives proper pretreatment and finish, but copper and bronze will not remain attractive unless properly finished either. Copper and bronze are so much more expensive than steel that their architectural uses are quite limited.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
February 2015
Q. I am doing an actual experiment involving which will rust--steel, copper, and bronze. I need to obtain the metals, but I am not sure how much/which form of each metal I need to get and exactly which type of each. Could you please advise? I was looking at onlinemetals.com/ to try to order.
Thank you!!
11th grader - Shannon, Georgia USA
March 1, 2015
A. Hello John. we appended your inquiry to a long thread which covers it rather completely and offers some other sources. For the purposes of such a test, I don't think it much matters what type of steel, copper, and bronze you get; go for the cheapest, but carefully retain full detail on what you get. What you'll be looking to get from this experiment is not so much the specific result as learning how to properly undertake such projects -- which will include documenting information as accurately as possible. Good luck.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
February 2015
Q, A, or Comment on THIS thread -or- Start a NEW Thread