
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Using fruits and vegetables to produce electricity
Q. My science project will try to prove that by inserting electrodes in lemons, oranges, potatoes, carrots and salt water, there is a possibility that one or more of these items will produce electricity and light a small lamp. I need to know if anybody can help me in this experiment by giving me some information as to what is the best way of doing this experiment.
Thalia B [last name deleted for privacy by Editor]6th grader making a science project . - Tarzana, California
2003
2003
A. You may be young to really understand much of this, Thalia, but a fruit or vegetable doesn't exactly produce electricity, it just provides a conductive liquid that completes the circuit.
To take it one step at a time, look at the diagram. As it is shown -- fruit or no fruit -- the bulb will light because there is a battery and a complete circuit. The current flows from the battery, through the blue wire, through the bulb, through the brown wire, through the left electrode, through the red wire, through the right electrode, and through the green wire back to the battery.
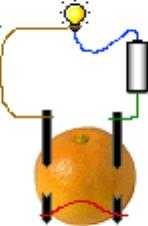
Now, for the second step, if the orange juice is a conductive liquid (electrolyte), which it is, the red wire can be removed and the system will still theoretically work because electricity can flow from the left electrode through the juice to the right electrode, so we still have a complete circuit. I emphasize "theoretically" because liquids do not conduct electricity anywhere near as efficiently as metal, and real flashlight bulbs require a significant amount of electricity; while some very small amount of electricity will flow, unless you have very large electrodes and extremely close spacing it will not actually be enough to light a bulb; it might be able to light an LED (light emitting diode) and surely will be able to turn on an LCD (liquid crystal display).
Next, for the third step, you can remove the battery and connect the loose ends of the blue and green wires together. Now you have a complete circuit, but you don't have a battery anymore so the light goes out.
Finally, make the electrodes out of two different metals (say copper and zinc) and the lamp will light again (theoretically). This is because two different metals put into a conductive electrolyte actually comprise a battery. In fact, that's what any battery is: two different metals with a conductive solution between them. So it's not that the orange "produces" electricity, it is that copper plus zinc plus orange juice as the conductive electrolyte make a battery.
In this case, the acidic juice allows a small amount of zinc and copper to dissolve in solution. But the zinc has a stronger propensity to go into solution so it drives the copper back out of solution. The net result is that copper dissolves from the copper electrode and deposits on the zinc electrode. Copper metal thus moves from the copper electrode to the zinc electrode (eventually completely coating it). Copper, like all metals (and all materials) has electrons. When the copper dissolves into solution it leaves some of its electrons behind on the copper electrode and dissolves into solution as a positively charged ion, but those electrons that don't travel through the solution with the copper ions, must travel to the zinc electrode through the wire. This movement of electrons is electrical current.
As I said, some of this will be over your head, but that's as simple as I can make it, and you can absorb what you can from it. Good luck.
In real commercially-sold batteries, the surface area of both metals is very large and placed very close together to maximize the electricity. The actual amount of electricity you will produce is very small and you will probably not be able to find even a penlight bulb that you can light with this little bit of electricity, but light emitting diodes (LEDs) require less electricity, so you may be able to light a very small one. The liquid crystal displays on electronic calculators use even less electricity (almost none) so you will be able to operate that if you can find one and (with a parent or teacher's help) connect your "orange battery" in place of the built-in battery.
There is a great youtube video on lemon batteries at:
youtube.com/watch?v=AY9qcDCFeVI

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
A. You can get a "scientific toy" called a "potato clock". It comprises a small LED display and a few pieces of wire that are attached to a piece of copper and a piece of zinc. You connect the other ends of these wires to the LED and put the copper and zinc into to potato. Lo and behold, the clock starts to go and will go for a go few weeks until the potato shrivels up. the clock will work with most fruits, such as apples, oranges, peaches, grapefruits etc, but it is not very good with vegetables such as brussel sprouts or cabbage!

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
2003
Q. Hello,
Thank you for taking this question and for your help. I am conducting a science experiment where I am conducting electricity with fruit. I cleaned a zinc strip and copper wire with 00 steel wool ⇦ this on eBay or Amazon [affil links] . Then I inserted the zinc and copper into fruit (lemon, lime and orange) about 1 inch apart. Next I hooked up a multi-meter to the ends of the copper and zinc and measured the electricity. I am sure that it had something to do with dissimilar metals as much as it has to do with the different acids in the different fruits. Do you have any input on why the electricity is conducted the way it is? Any help would be great. . Thank you for your help.
Colton C [last name deleted for privacy by Editor]student - Garland, Texas
2003
A. Remember that every explanation of things is our physical world is only a simplification, an idealization, a helpful way of viewing things.
So it is difficult for people to answer your questions when we don't know what grade you are in, Colton, because the explanation needs to be a simplification that you can understand at this point in your education, but not so simplified that you learn nothing new from it.
The salt in the ocean never settles out, not because the ocean keeps moving, but because the water can dissolve that much salt. Similarly, if you put a small pinch of sugar in your tea, it will dissolve. Even after you stop stirring and let it sit for hours or days, that sugar will never ever settle out. But if you put several spoonfulls in, the 'excess' is not really dissolved, it is just swirling around and it will slowly settle. There is an equilibrium point between the solid sugar settled at the bottom, and the dissolved sugar. As a molecule of sugar comes out of solution, another one dissolves into solution.
It is similar with metals and acids: a small amount of metal dissolves into solution and then there is an equilibrium. But if you put two different metals into solution, what happens is the more active metal (zinc) bumps the more noble metal (copper) out of solution. Eventually, the zinc rod will become completely covered with copper and there is no more zinc exposed and the 'battery' will go dead. But until then, as the positively charged Cu++ ions move through the juice from the copper rod to the zinc rod, electrons are forced to move through a wire from the copper rod to the zinc rod to balance that movement of charge.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2003
|
Q. Which fruits and vegetables are most likely to light a light bulb? How does the acidity level effect this? I am doing an experiment to combine the two, what part acidity level plays in electricity. What help can you give in performing this experiment? I am in 9th grade and performing this experiment for a science fair. Jamie S [last name deleted for privacy by Editor]student - Marysville, Pennsylvania 2004 Q. Hi, I as well am conducting an experiment involving electricity through fruits. I know I must use the zinc and copper. Is there a particular way these strips should be connected to the alligator clips when measuring the voltage of the electricity produced? Missy B [last name deleted for privacy by Editor]Student - Brownsville, Texas 2004 Q. I am a student from XIIth standard. I am doing a project on "Electric charge in fruits and vegetables" it would be a great help if you could kindly give me some information regarding the theory behind the process .how it works? how it conducts? thank you, kasturi Thank you sir for your kind assistance but I regret to inform you that I have gone through your homepage regarding my project on "electric charge in fruits and vegetable and found the information insufficient .i wrote the last letter hoping that you could elaborately explain the procedure and the theory behind electric charge in fruits and vegetables. and also how to measure the resistance offered and current flowing through the circuit . I'll be highly obliged if you could reply to my inquiry at your earliest. kasturi b [last name deleted for privacy by Editor]student - guwahati, assam, India 2004 |
A. Jamie: Pick some fruits and vegetables; lemons, limes, oranges, potatoes, and carrots have already been suggested; you could also try grapefruit, pineapple, and cucumber. Do the experiment and tell us your findings before you ask that they be explained.
Missy: you just put one lead of the meter on the one type of metal and the other lead on the other metal, and don't let the two metals touch.
Kasturi: A multimeter, sometimes called a voltmeter or a VOM ( ⇦ this on eBay or Amazon [affil links] ), will easily measure the resistance of the fruit or vegetable in question and the current flowing through it. It will also measure the voltage produced. They are available for $10-$15 from electronics stores or on-line. Good luck with the project.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2004
Q. I would like to know if a penny can be used in a science experiment as a copper electrode to generate my potato battery to work? Thank you so much please reply soon
Your dearest,
Cassie M [last name deleted for privacy by Editor]student - Mannheim, Germany
2004
A. In the USA pennies made after 1982 are copper plated zinc, not solid copper, and they would not be a really dependable electrode because they could lose their plating. If your penny is solid copper (before 1982) it should be better. But a piece of copper wire would be best because copper wire is made of very very pure copper.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2004
Q. I tried out an orange, a potato, and an apple. none of them made the light bulb react. I used one nine volt battery, copper conductors, and a test light. Why didn't the light react when I placed them correctly?
Patrick L [last name deleted for privacy by Editor]Max Abbott Middle School - Fayetteville, North Carolina
2004
A. Patrick. Did you wire it per my drawing above? Did it light when you put the red wire back in place? Remember that a conductive liquid cannot carry nearly as much electricity as a wire, and may not carry enough to light that bulb. But try again with the electrodes as large as practical, and nearly touching.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2004
by Steve Spangler
on AbeBooks
or eBay
or Amazon
(affil links)
Q. I am doing a science project on which fruit will conduct electricity the best. I am using an orange, apple, lemon, grapefruit and a pear. How do I do this experiment? Any suggestions would be helpful.
Thank you,
Scott C [last name deleted for privacy by Editor]- Ogden, Utah
2004
Ed. note: There is a great youtube video on lemon batteries at: youtube.com/watch?v=AY9qcDCFeVI
A. Scott, I am presently assisting my Daughter a 7th grader regarding her lime fruits project. We were able to utilize the carbon and the zinc rod from a discarded size "D" battery and insert it in the lime far apart. Our reading was 1.1 volt using a small digital multimeter, the resistance reading was 2000 ohms taken from analog voltmeter. Finally, we connected two limes in series and we got a reading of 2.2 volts meaning that you can increase the voltage indefinitely and the possibility that it can produce enough current to activate a light emitting diode. P.S. you can purchase LED diode from Radio Shack or from unused electronic toys.
Raymond P [last name deleted for privacy by Editor]- Chicago, Illinois
2005
![]() I like your approach of using the carbon and zinc from a battery, Raymond. It helps the kids understand batteries and electricity without having to introduce the new concept of using copper instead of carbon while they're still trying to learn the first concept.
I like your approach of using the carbon and zinc from a battery, Raymond. It helps the kids understand batteries and electricity without having to introduce the new concept of using copper instead of carbon while they're still trying to learn the first concept.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2005
Q. I wish to know for my project which of these fruits will produce the most energy to a light bulb. an apple, peach, lemon, or a mango.
Paul L [last name deleted for privacy by Editor]student - E. Petersburg, Pennsylvania
2005
A. Paul, what you are no doubt supposed to do is find out the answer by doing an experiment. Watch the youtube video on lemon batteries and do the same stuff with the other three fruits. But you must use words very carefully in science: are you sure that you mean "energy"? Good luck!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. I am doing a science project on which fruit (lemon, mango, kiwi, and apple) will act as a conductor in a simple circuit the best (producing the brightest light). In making my simple circuit I used wire, a flashlight head, a fruit, and 2 D batteries. I had hoped there would be some result in at least one fruit but none produced any light.I'm in sixth grade.
Kimberly C [last name deleted for privacy by Editor]student - Valleyford, Washington
2005
A. Kimberly. current is flowing, but not enough. Get a bulb from a 1-1/2 volt penlite. Although you weren't able to light the 3v bulb, you may be able to light the smaller one. If not, try more batteries, or a bigger surface area for your electrodes (by crunching up a bunch of wire instead of exposing just a quarter inch of it. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. I have just completed an experiment on fruits and vegetables conducting electricity using pH paper and a volt meter. I found out that the fruits like lime, orange which are acidic conduct electricity the best. But I had found that 2 vegetables, (potato and tomato) had low acidity but conducted electricity well. Why is this the case?
Joel N [last name deleted for privacy by Editor]5th grade. - Cabin John, Maryland
2005
2005
Q. Hello,
I have a few questions concerning the experiment about conducting electricity and the use of fruits and vegetables.
1st. Where can you get zinc electrodes?
2nd. What if any fruits or veg's are NOT good conductors of electricity?
3rd. When connecting the light bulb what is the best to use?
Any help you could provide would be greatly appreciated.
Thank you,
Jim
- Tacoma, Washington
A. 1. You can get solid "zinc anodes" from a boating store, Jim. You can also get zinc from a carbon-zinc dry cell battery with some help from the science teacher. The easiest thing to find might be galvanized roofing nails; they are not solid zinc, they are only zinc coated, but for this experiment they will be good enough.
2. Any fruit or vegetable that is too dry or is not acidic would probably be a poor choice. So would a fruit or vegetable like brussel sprouts, cabbage, or broccoli that isn't solid. Lemon, limes, and grapefruit are probably the best.
3. The currents are very small, so a tiny LED would be good. Even better, a liquid crystal display. Any copper wire would be fine.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2005
(you are on the 1st page of the thread) Next page >
Q, A, or Comment on THIS thread -or- Start a NEW Thread

