
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Rock hard scale problem in aluminum processing
Q. Looking for ways to Reduce / Eliminate 'rapid scale buildup' in Drain Lines of Rinse Tanks following Black Oxide Treatment of Iron and following Caustic Etch of Aluminum.
Both Treatment Processes involve strong Sodium Hydroxide materials and these 'salts' attach aggressively to the walls of the drain lines, regardless if constructed of Steel or PVC.
This requires frequent A) Replacement of Drain Lines {Where Accessible} and/or B) Aggressive Mechanical Clean-out.
Can anyone share Means to address this problem?
MANY THANKS
- Mishawaka, Indiana
January 7, 2021
January 2021
A. Hi Lyndon. We appended your inquiry to a generally similar thread where some ideas have been offered...
Regarding black oxide, EPI / Electrochemical Products Inc. [a finishing.com supporting advertiser] has an article at
epi.com/blog/post/epi-how-to-clean-out-your-black-oxide-tank/
which suggests that some black oxide formulations can hold more iron, thereby limiting the problem.
This thread offer some ideas for the aluminum etch sludge, including addition of sodium gluconate.
It may be impractical for rinse tank drain lines, but if possible, keep recirculating the sludge and it will not attach and harden. Anything that keeps the sludge moving is key.
My own experience suggests that such sludges are NOT actually a scale; rather, when they first form they are rather light and fluffy well-hydrated precipitates. But when they are allowed to sit undisturbed, the heavier-than water sludge components quickly drive out all the interstitial water, leaving a rock. Take a plastic bottle full of the etch solution and I think you'll see it settle into a hard precipitate at the bottom and clear liquid above. Bu if you patiently invert the bottle many times, the 'rock' will be gradually washed back into solution. I have suggested on this thread a way that such sludge can be handled with a paper "gravity" filter, but no one has actually tried it to my knowledge :-)
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Alkaline Pickling filter housing blockage
May 15, 2021Q. I have come across an issue with our alkaline pickling tank filter housing becoming blocked
Im not sure how this has happened if this was left off and the contents somehow solidified ?
This isn't a sludge and is completely solid and near impossible to remove, is there any chemical I can add to somehow free this up to become more manageable
The chemicals used in the pickling bath are
- 30% caustic soda ⇦liquid caustic soda in bulk on
Amazon [affil link]
( 50 g/l target in bath)
- sodium 3-nitrobenzenesulphonate 7%
- trisodium nitrilotriacetate 7% (15 ml/l target in bath)
The pickling is to remove any scale or oxide present used in the pre-treatment of plating Al with Zn/Ni/Sn
Setup is
Degrease
Rinse x 2
Pickling
The only possible contamination would from our alkaline degrease and this would be minimal with a double intermediate rinsing stage
Any ideas on what this is and secondly how I can remove this chemically ?
Any help would be appreciated
- Derry Ireland
A. Hi Ryan. This is not an unusual problem. Read on for ideas on how to avoid it and how to deal with it. Mileage may vary, but I feel the best approach, if possible, is recirculation if that is possible even at a greatly reduced rate, and gradually 'washing' that aluminum precipitate onto your filter cartridges or plates.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
May 2021
May 20, 2021
Q. Hi Ted,
Thanks for the response
I have been looking into ways of trying to avoid this - the problem I'm having is that its completely solidified into the bottom of our stainless steel tank
This seems impossible to remove and looking to find a way to do this.
Looking at the chemical makeup of our tank and the complexing agent I'm going to up the concentration going forward to avoid this.
Could this be happening because the Al ppm after etch is getting too high and the solution is getting spent/saturated?
This was also an issue with blocked filtration pipes; it wasn't solid and was essentially caked on - this was like a sludge - this was removed by hand !
Our pickling tank is 2938 L and looking at a few responses these look fine for a small scale, any ideas on how I could implement this on a big scale
We are changing filters every week also to try and remove as much as possible !
I have sent photos of the issue but can't see them on the thread itself
Any further ideas would be greatly appreciated.
- Derry Ireland
A. Hi Ryan. There have been suggestions about complexing agents on this thread, and hopefully another reader will have further suggestions; but sorry,I don't. I have seen this problem in aluminum capacitor manufacturing, and in precious metal slurries applied to catalytic converters, and in aluminum etching solutions, but I am not a chemist experienced in exactly what if any chemical reactions encourage it or will deter it.
My observation is that what starts as a light fluffy sludge gradually turns into a rock, not as a result of a continuing chemical reaction, but because -- for whatever reason -- these particular sludges slowly settle in a way that eventually pushes the water out, like in a winning game of Tetris :-)
I am 75% confident that your 2938 L tank can be cleared of that rock over the period of a couple of hours through the recirculating approach I sketched back in 2003, with a pressurized lance and using whatever filter you have available -- it's just that a paper gravity filter or paper vacuum filter is an ideal way to get rid of huge amounts of such sludge whereas it may take an unaffordable number of cartridges to capture it all.
Sorry I see no pics from you; please attach them to an e-mail to mooney@finishing.com.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
May 2021
⇩ Related postings, oldest first ⇩
Q. I am looking for a complexing agent for aluminum to use in one of our etching baths. The pH of the bath is about 2 and right now we are getting AlF3 scale (rock hard) formed in the bottom of our test tank. This bath will be used for cleaning and etching aluminum alloys. EDTA is an obvious complexing choice, but I have doubts on how well it would hold aluminum at pH 2.
Terry Tomt- Auburn, Washington, USA
2003
A. Dear Terry,
Look for aluminum fluoride is high 19.37. So you can use 5SSA 5 sulphosalycilic acid with log k=29.
Hadi Khosravi- Tehran, Iran
2003
2003
A. Try sodium gluconate.
- Goleta, California
Rest in peace, Ken. Thank you for your hard work which the finishing world, and we at finishing.com, continue to benefit from.
![]() Thank you for the responses. I am concerned that sodium gluconate is made to work in alkaline pH region and not in acid as my case is. I will look into the salicylic acid for sure. Also I will research the gluconate.
Thank you for the responses. I am concerned that sodium gluconate is made to work in alkaline pH region and not in acid as my case is. I will look into the salicylic acid for sure. Also I will research the gluconate.
- Auburn, Washington, USA
2003
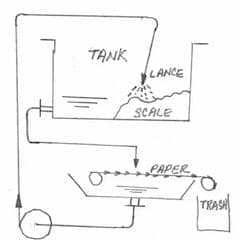
A. It may be of only academic interest to you, or no interest, but this 'rock hard' sludge might only become rock hard when it's allowed to settle and squeeze out the interstitial water. If you can keep it moving, it may never harden. Even after it has hardened, though, I think you can remove it by getting it back into solution via patient washing, and then filter it out. I have seen a number of aluminum sludges/slurries that do this.
I haven't actually tried it, but I'll bet you can clean it out by using an automatic paper filter and recirculating water through the tank, directing the output at the scale

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Avoid AlF3 rock hard scale formation in acid etching
Q. I am looking for advise how to avoid AlF3/AlPO4 scale in our hydrofluoric/phosphoric acid etching baths. The baths are used to etch aluminium alloys. We currently have to shut down the production every 3 months to carry a "dig out" with drilling pneumatic hammers and remove all the AlF3/AlPO4 scale (rock hard)from the walls and bottom of the etching tanks...
What Al complexing agents do you recommend other than EDTA? EDTA is the most obvious choice but don't know if it would work at pH 2 and represents a serious problem to the environment. What could we do to avoid the Al sludge to become rock hard? Continuous stirring? Rising the temperature?Any practical solutions?
Any help would be greatly appreciated. Thank you :-)
- Peterborough/UK
February 10, 2018
A. Hi Nuno. Any chance of hooking up an automatic paper filter, either for continuous use or for periodic cleanup per the sketch on this thread we appended your inquiry to?
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
February 2018
Q. Hi Ted, thank you for your prompt answer. This thread has been the most informative...
Do you know if actually Ted's idea had been successfully put into place? Wouldn't the paper filter be quickly deteriorated for continuous use due to the very low pH of the etch bath?
One last question: do you know if using chelating agents such the ones mentioned would work and would it be economically feasible? Are there any new ones you're aware of?
Thank you once again and congratulations on this knowledge exchange platform. Wish there were much more...:-)
Kind regards,
- Peterborough, Cambridgeshire, United Kingdom
February 11, 2018
A. Hi Nuno. If you collect a plastic bottle full of your slurry, I think you'll discover that it settles out in a few hours to a rock hard sludge and a decantate ... but that if you turn the bottle over and back a hundred times or so the 'rock' gradually washes back into a slurry. If your aluminum 'scale' acts as I describe, I think it is possible to continuously filter the solution or, lacking that, to recirculate the decantate to wash the 'rock' back into a slurry for periodic filtration.
Automatic paper filter systems use disposable newsprint which unrolls onto a continuous belt of stainless steel mesh. So the fact that newspaper isn't particularly acid/caustic resistant shouldn't matter -- although the rest of the filter parts would have to hold up to the solution. I haven't tried it.
I am not a chemist and cannot comment on the complexing suggestions offered by Hadi, Ken, and Terry.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
February 2018
![]() Thanks Ted, your help was precious! I'm going to study and explore all these ideas. I'll keep you posted :-)
Thanks Ted, your help was precious! I'm going to study and explore all these ideas. I'll keep you posted :-)
Hopefully with good news...
Kind Regards,
- Peterborough, Cambridgeshire, United Kingdom
February 12, 2018
Q, A, or Comment on THIS thread -or- Start a NEW Thread