
-----
Researching Faraday's Law
Q. I am doing my GCSE [General Certificate of Secondary Education] course work for chemistry and I wanted to know EXACTLY what Faraday's Law and Faradays constant IS? word for word in a description and formulae so that I can understand it. PLEASE!
Anna Marie Baines- England
2003
A. Hi Anna. Faraday's Constant is 96,485 ampere-seconds per mole (or coulombs per mole).
But as for his law, "Word for word" and "so that I can understand it" is sometimes a contradiction because many science "Laws" are written tersely and intended for those highly skilled in that science. Wikipedia says:
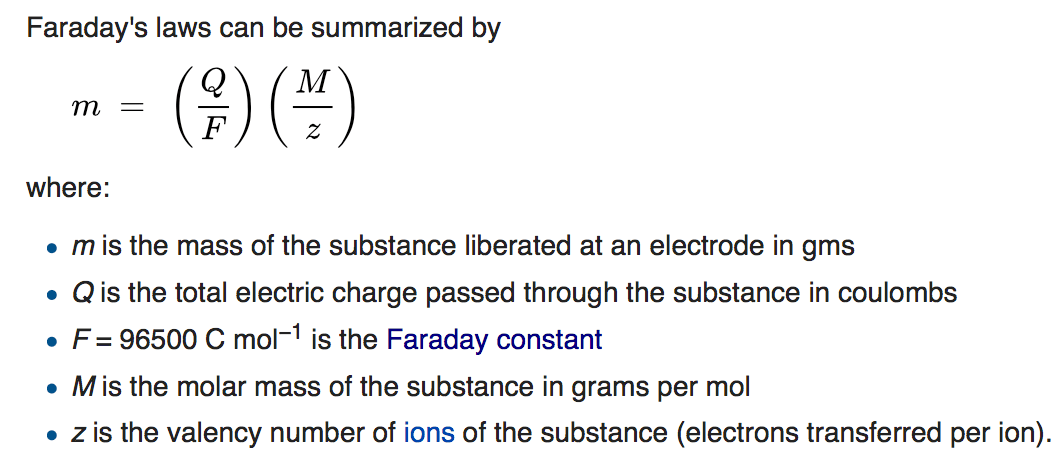
As someone from the plating industry who uses Faraday's Law all the time, I have tried to slowly and carefully explain it from an electroplating perspective at "Faraday's Law of Electrolysis for Plating". I hope it helps!

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
2003
Q. If I want to measure or control the weight or thickness of material which is getting electroplated on the surface of the cathode, what should I do? How can I relate the time of electroplating and applied current to the thickness or weight of deposit or vice versa?
Thanks,
Curious student - Bath
October 14, 2008
Q. Hello Ted,
your article is very helpful to me.
Thank you for that.
I have a question.
How we can calculate quantity of H2(g) in electroplating bath?
- Istanbul, Türkiye
October 5, 2011
A. Hi, Ömer.
Sorry, but I don't believe that it is practical to calculate the efficiency of electroplating baths; rather it is empirical knowledge. A copper, nickel, or gold plating bath may approach 100% efficiency when operating at a high concentration and when the plating is conducted extremely slowly, and will be about 90% under normal operating conditions, but the efficiency will drop at higher plating rates or lower concentrations.
Picture what is happening at the cathode: electrons are arriving there through the external wiring circuit, and must be satisfied. If an ion of metal has migrated through the boundary layer and is available for reduction, it will be reduced and deposited. But if no metal ion is there, the available electrons will reduce a hydrogen ion instead. A chrome plating bath involves a very complex series of reactions and typically operates at about 12% efficiency, but most plating is much more efficient -- 90% is not at all unusual, and virtually 100% if often possible.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
October 5, 2011
Q. Hi There,
Could you please tell me what effect the plating time (the time period in which electroplating was conducted over) would have on the amount of copper (or any other metal) that can be plated during electroplating? The results of the experiment showed that as the time period increased so did the mass of copper plated but I am confused why this occurred?
Many Thanks.
- Brisbane, QLD, Australia
March 14, 2018
A. Hi Rosie. We appended your inquiry to a thread which addresses it. In brief, the mass of copper plated is directly proportional to the plating current X the plating time. See our "Faraday's Law of Electrolysis for Plating" for a complete explanation. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
March 2018
Q, A, or Comment on THIS thread -or- Start a NEW Thread