
Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
Can gold be dissolved in sulfuric acid alone?
Q. Dear All,
I have a wager to settle to with a friend and so quite a basic question really, I would like to know very simply if gold can be dissolved in sulfuric acid or is it really only aqua regia that can do this?
Thank you for your help in advance and have a lovely day.
Best regards,
Patrick [last name deleted for privacy by Editor]WMRC - London, UK
2002
A. No winner; donate the kitty to charity :-)
Gold cannot be dissolved in sulfuric acid to my knowledge; but it can be dissolved in a number of things other than aqua regia, including cyanide, citrates, and sulfites.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
2002
2002
by C.W. Ammen

on AbeBooks
or eBay or
Amazon
(affil links)
A. No. The only acid that dissolves gold is aqua regia. How much did you win?
Ted is of course right. Gold will dissolve in cyanide and citrates but, as far as I am aware, only with the application of an electric current.

Trevor Crichton
R&D practical scientist
Chesham, Bucks, UK
August 2014
![]() Thanks for correcting me gently, Trevor. Surely I was guilty of mental sleight of hand when I tried to equate using electricity and cyanide to dissolve gold with dissolving gold by simple immersion in aqua regia.
Thanks for correcting me gently, Trevor. Surely I was guilty of mental sleight of hand when I tried to equate using electricity and cyanide to dissolve gold with dissolving gold by simple immersion in aqua regia.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
! Trevor Crichton, Gold dissolves in cyanide solutions without application of electric current. Witness use of weak cyanide solutions for recovery of gold by heap leaching.
Fuzail SiddiquiIndependent Consulting Geoscientist - Markham, Ontario, Canada
September 16, 2014
March 7, 2012
"Can gold dissolve in sulfuric acid alone?"
A. The answer is yes, and no. According to a 1940 patent #2185858, gold or palladium are stripped from a copper based substrate by making the parts anodic in a fairly concentrated sulfuric acid solution. According to the patent, persulfuric acid is produced at the anode surface, which momentarily dissolves the gold. Once away from the anode, the dissolved gold immediately reduces to a powder. When all the gold has been stripped from the parts, the current goes to zero. All the gold powder settles to the bottom and is then collected.
I guess the keyword in the original question is "alone." The gold does dissolve, using no other chemical than sulfuric, but it does require electrolysis.
I have used this stripper many 100s of times for gold recovery from plated objects. It works for many substrates besides copper, even aluminum.
- Nevada, Missouri, USA
December 8, 2012
A. Sulfuric acid can dissolve gold, but it is a very dangerous process as the sulfuric acid has to be concentrated and heated to the point sulfur dioxide gas is fumed off, silver or gold will dissolve gold will form a gold persulphate soluble in the very concentrated acid, upon dilution the gold will precipitate out of solution as a powder which can be washed and refined. (Another terribly dangerous process) (never add water to concentrated sulfuric acid a steam explosion of hot acid can go everywhere)(always add acid to water slowly to dilute), concentrated boiling sulfuric acid can disable a man for life,and a little bit of gold is not worth the risk.
Another way gold is dissolved in concentrated sulfuric acid is by making the gold an anode the persulphate that forms at the anode will dissolve the gold into solution as gold persulphate, but as these gold ions migrate towards a lead cathode the gold is no longer a persulphate in the more dilute solution away from the anode, so the gold will precipitate in the cell, again gold is recovered by dilution of the acid (see warnings above), this impure gold is washed and then refined
People who would re-plate silver would use a process similar to this to remove the silver from copper or brass items (the concentrated sulfuric would not attack the copper or brass (as long it was kept concentrated), they would use electrolysis and about 5% HNO3 added to the electrolyte to strip the silver plate, silver plated article was on the anode.
To dissolve gold or platinum there are several ways to do this besides aqua regia.
Aqua regia is not the only thing strong enough to dissolve gold and form a gold chloride solution.
HCl and NaCLO 5% sodium hypochlorite (household bleach)
⇦ bleach/sodium hypochlorite in bulk on
eBay
or
Amazon [affil links]
will also dissolve gold and form gold chloride
HCl and 30% H2O2 Hydrogen peroxide will also dissolve gold to form gold chloride
There are other oxidizers that will work.
There are even salts that when fused with gold can make the gold water soluble when the fusion is dissolved into water.
- Grants Pass, Oregon, USA
Q. My dog ate my gold earring and has not passed it after one week. I understand that he has a pocket at the bottom of his stomach for hard to digest materials to linger while his stomach acids break them down. My question is, will my dog's stomach acids dissolve the gold earring?
Kathy M [last name deleted for privacy by Editor]- West Palm Beach, Florida
January 20, 2009
Hi, Kathy. You might talk to a vet; I don't think your dog's stomach has the capacity to dissolve gold.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
![]() Thank you for your response. Seems like a good blog, although most are probably trying to stay warm rather than typing with cold fingers!
Thank you for your response. Seems like a good blog, although most are probably trying to stay warm rather than typing with cold fingers!
- West Palm Beach, Florida
January 22, 2009
Hello Ted,
Thought I would let you know the outcome of my dog swallowing the earring. Yesterday my dog walked out into the kitchen to exactly where I stand to dish up his food and left my gold earring on the floor for me to find ! I happened to be watching him out of the corner of my eye and he just set it down and walked away. Since I had cleaned the house from top to bottom, we are for sale down here, the only thing I did not do was check "in his bed" ! I don't know where it came from, but I would have to guess that he had taken it back there to play with. In perfect condition, both the earring and the dog ~..~ Thank you for answering my inquiry, stay warm. Best Regards.
- West Palm Beach, Florida
January 26, 2009
January 27, 2009
Hi, Kathy. Thanks for sharing the happy ending.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Interesting reading about the dog.
When you say it brought put the ear-ring on the kitchen floor, do you mean that it carried it in in its mouth, and put the ear-ring on the floor ?
or ... he did his Doggy Duty on the kitchen floor, and his special gift contained the ear-ring ?
I had food poisoning twice in 2002. I learned more than I would have liked about stomach acid.
What I was looking for was info about dissolving gold, what types of solvents & acids work.
Lots of good info here !
- Santa Rosa, California
November 3, 2009
Q. Hi,
I would like to know can I dissolve gold using potassium iodide (KI)+ iodine (I2)+ H2O?
Regards
Adriano
- Jakarta, Indonesia
March 2, 2012
A. Yes, KI + I2 is a common gold stripper, especially in the electronics industry. These chemicals are quite expensive, but there schemes which precipitate the gold and rejuvenate the solution for further stripping.
Chris Owen- Nevada, Missouri, USA
March 7, 2012
Q. (1) Will sulfuric acid remove solder from boards and/or pins? Last year I put a lot of small board pieces in HCl and left them too long I guess as the gold was removed as well. I filtered out the cut off pins, but have done nothing else to the solution.
(2) Is it too late to drop out the gold? If not
(3) How can I do it?
Thank you
Buddy

Buddy (The Junk Bunny) Norville
Munday Recycling Center - Munday, Texas
November 27, 2015
Will sulfuric acid remove solder from pins and boards? Without removing gold?
Q. I started recycling in our town five years ago. About the same time I started recycling electronics. I've always been a scrapper so I just added electronics to the heap. I am presently frustrated with the price of everything being so low, my backyard is cluttered with scrap metal and junk electronics. I've been accumulating e-scrap all this time, with many boxes of stripped boards, hard drives, power supplies, etc. At the same time I've been watching videos, trying to learn how to do things. I've found that to be frustrating as well. Questions arise as I watch but answers are not quick to come back to me.
This site appears to be better in that respect. I have enjoyed reading the dialog. So here I am, ready to learn.

Buddy Norville [returning]
Hobbyist, recycler - Munday, Texas U.S.A.
November 29, 2015
Q. Hello guys. I'm 12 but I'm going to invent a robotic super armor that is strong against most things, but I can't figure out what material does not dissolve in anything and is as strong as carbon. In 30 years you'll see me in the news.
Live Long
- Taylorsville, Utah, U.S.A
December 3, 2015
![]() Hi Yuuto. Despite your suggestion that we should live long, I'm probably not going to hold on for 30 more years -- could you please move your timetable up by a few years? Gold is very corrosion resistant but not too strong; high strength stainless steels don't dissolve in much though. Good luck.
Hi Yuuto. Despite your suggestion that we should live long, I'm probably not going to hold on for 30 more years -- could you please move your timetable up by a few years? Gold is very corrosion resistant but not too strong; high strength stainless steels don't dissolve in much though. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. I have about 500 g of gold alloy and I don't know the metals. I have checked and it's below 9k so the machine could read it. I want to recover using sulfuric acid and I don't want to lose the gold. Can I get rid of the metals for this process? How is it if I make the gold as cathode and lead anode? Thanks.
Omer Abdi- tokyo, japan
February 5, 2016
Q. I believe that I have dissolved gold and antimony in sulfuric acid. If so, can I selectively precipitate gold with Stump Out? If yes, how?
Robert SchlichterR and D - Newbury Park. California USA
June 15, 2016
A. You could try adding urea. That precipitates gold from aqua regia. Or, zinc powder, or steel wool. Either would dissolve producing hydrogen and the gold would deposit out.

Dave Wichern
Consultant - The Bronx, New York
June 16, 2016
A. No offense, but I must disagree with Dave. Urea will not precipitate gold from an aqua regia solution. The most common gold precipitants are some form of sulfite, such as sodium sulfite, sodium bisulfite, or sodium metabisulfite. Less common but still viable gold precipitants are ferrous sulfate
⇦ this on
eBay or
Amazon [affil links] or oxalic acid.
If the worker uses too much nitric acid in the aqua regia, the sulfite will react with the excess nitric before any gold will precipitate with the sulfite. Urea is often used first to tie up the excess nitric so that the gold will precipitate when the sulfite is added.
It is better to use sulfamic acid to eliminate the excess nitric. Urea is a poor choice because, when there is a lot of excess nitric, crystals (urea nitrate, I think) will form in the solution. With sulfamic acid, the reaction products are sulfuric acid and nitrous oxide (laughing gas). Also, sulfamic acid decomposes the nitric, whereas urea somehow prevents the nitric from interfering with the sulfite but doesn't decompose it (as I understand it).
No matter what you use, a good fume hood is a must.
- Nevada, Missouri, USA
June 17, 2016
June 19, 2016
![]() I stand corrected, then. Sorry if I confused anyone. I used to analyze the wastewater from a company that did gold refining (very highly mineralized - after the evaporation that came with the digestion, on cooling half of it would solidify) and they gave me to understand that they used urea. I guess I misunderstood.
I stand corrected, then. Sorry if I confused anyone. I used to analyze the wastewater from a company that did gold refining (very highly mineralized - after the evaporation that came with the digestion, on cooling half of it would solidify) and they gave me to understand that they used urea. I guess I misunderstood.
My impulse would be to use steel wool.

Dave Wichern
Consultant - The Bronx, New York
Q. I have now got all my foils ready to refine only to find that I cannot now obtain any acidic liquids. I need help in how do I remove my foils, and how to dissolve them without the products needed? I cannot even get any borax ⇦ this on eBay or Amazon [affil links] for my end product. Please help with simple easy answers as I am no chemist and things take longer to sink in. Thanks.
fred bolamhobby but work in recycling industry - england
May 8, 2017
May 2017
? Hi Fred. You are asking how a person with no chemical knowledge, and unable to obtain either borax nor any acids, should dissolve gold, a metal prized for millennia for many reasons including being virtually non-dissolvable? But what do you mean by your "foils"? Thanks.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Q. Okay guys, so I'm new to this whole gold refining thing. Kind of jumped in head first. I started off using hydrofluoric acid; it dissolved everything down to a powder. After being washed to heat it up to melt off everything other than the gold takes too long, so I got a bright idea: sulfuric acid (just because I found it in the woods) and so now I have my powder mixture mixed with sulfuric acid and I want to make sure I'm not going to blow myself up with this mixture of powder and sulfuric acid. I'm sure there is probably some residue of this, probably some residue from the hydrofluoric acid ... and also will the sulfuric acid dissolve away everything leaving just the gold? Thank you very much. You have a blessed day. Blessed Be. Thank you for your help
Joshua Cook- Lakehills Texas USA
May 6, 2018
Hydrofluoric Acid
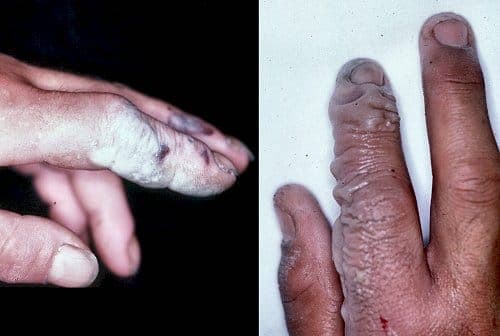
"HF burns, not evident until a day after"
by Dr. Charles Eaton
(http://www.handcenter.org/resumee/resumee.html)
A. Hi Joshua. I don't know much about gold refining, so we'll have to see if some other reader helps you, or you can search the site for many other threads on the subject. But you are dealing with very dangerous stuff in HF. Many professional platers who hardly give a second thought to most acids or hexavalent chrome or cyanide are quite frightened by hydrofluoric acid. You are also possibly even dealing with complete unknowns (just because a drum you found in the woods is labeled as originally containing sulfuric acid doesn't mean that's what was in the drum when you found it).
Regards,
.
Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
May 2018
Q, A, or Comment on THIS thread -or- Start a NEW Thread