
-----
Required solution temperatures for electroplating and electroless plating
Q. A fairly simple question - does the solution you use for electroplating need to be a certain temperature? (I'm doing gold on silver for jewelry purposes)...
Barbara Skeggs- Sydney, NSW, Australia
by Reid & Goldie
-- hard to find & expensive; if you see a copy cheap, act fast!

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Barbara. Yes, but what that temperature should be depends on a lot of factors including the general chemistry, whether you seek a bright or matte deposit, etc. In general, suggested plating temperatures for various plating solutions could be anywhere from 60 °F (some organic brighteners decompose at higher temperatures) to about 160 °F. The vendor of the plating solution should be able to give you the optimum operating temperature.
Typical cyanide silver plating solution temperatures are 75-90 °F. Non-cyanide proprietary silver plating solutions (which would be safer and far preferable in a non-industrial setting) must be per the vendor suggestion. Gold plating solutions are highly variable, being of alkaline, neutral, or acid types with suggested temperatures running the full 60 °F to 160 °F gamut. Good luck.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Electroless Nickel: Temperature vs. Speed of Deposition
Q. Can you direct me to a reference that will provide the rate of metal deposition as a function of temperature, all other parameters remaining constant? I am particularly interested in how temperature affects the deposition rates of electroless nickel, though the rate for current driven plating vs. temperature is also of interest. Graphs or equations would be nice to have.
Thank You,
electro-optics - Ann Arbor, Michigan, USA
January 9, 2014
A. Hi Don. For the Electroless Nickel, a great reference would be "Electroless Plating" by Mallory & Hajdu.
For alkaline baths it offers the following graph --
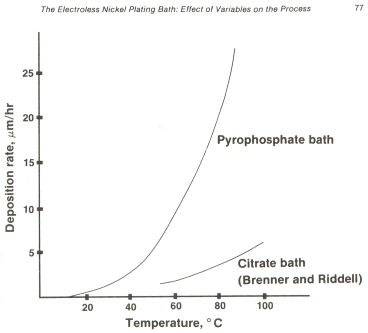
Obviously, the higher the temperature the better, except that spontaneous plateout can result from over-temperature.
Electroplating is very different though. For electroplating, the deposition is directly proportional to amp-hours applied (per Faraday's Law), and nothing else (including temperature) matters in that theory. But, in reality, higher temperatures offer greater ion transport rates in the bulk of the solution as well as across the boundary layer. And the reason ion transport rate matters is that the electrons on the cathode will reduce something . . . if insufficient metal ions are present, the electrons will be wasted liberating hydrogen from the water in the solution. How much metal is actually deposited compared to Faraday's prediction is called the "efficiency" of the plating bath, and for some plating solutions it will approach 100% at low plating rates and high temperatures, but will drop off at higher plating rates and lower temperatures.
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
January 2014
![]() Ted:
Ted:
You have been quite helpful. The reference, and especially the graph, tell me much of what I need to know. Your text helps a bunch, too.Thank you for the time and interest you have given to my problem.
Cheers
electro-opitcal science - Ann Arbor, Michigan, USA
January 10, 2014
Q. Ted:
The graph you have provided of the plating deposition vs. temperature for pyrophosphate bath electroless nickel is, for my purpose, gratifyingly steep. However, what parameters beside temperature may be adjusted to increase dP/dT? The slope is marginally sufficient to do what I think can be done. A steeper slope would help. It seems likely there are additives or the like that may enhance the slope. Are you aware of any such things that will help?
Thank you,
electro-optical engineering - Ann Arbor, Michigan, USA
February 3, 2014
by G. G. Gawrilov

on eBay or Amazon
or AbeBooks
(affil link)
Sorry, Don, that's not within my area of expertise. But if no one else answers, and you don't find the answers in Mallory & Hajdu, another good reference is Gawrilov's "Chemical (Electroless) Nickel Plating."
Regards,

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
February 2014
![]() Ted:
Ted:
Thanks, again, for your much needed help and the good references. I will read up a bit better and then run the problem through the analog computer. That is, do the experiment and see if I can solve the problem the cut and try way. This is, of course, the final arbiter of these things.
Cheers,
electro-optical engineering - Ann Arbor, Michigan, USA
February 4, 2014
Q, A, or Comment on THIS thread -or- Start a NEW Thread
