Curated with aloha by
Ted Mooney, P.E. RET

The authoritative public forum
for Metal Finishing 1989-2025

-----
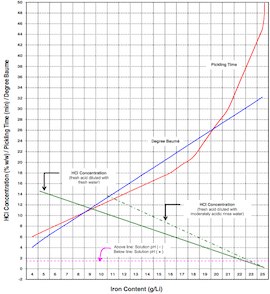
What Limit to Use for Iron Content in HCl Pickling
Q. SIRS,
IN OUR PICKLING BATH MEANT FOR STEEL TUBES, WE USE HCl SOLUTION. DUE TO RUSTY TUBES, IRON CONTENT IN THE BATH INCREASES. I WANT TO DRAIN OUT THE PICKLING SOLUTION ONCE IT GOES BEYOND A CERTAIN LIMIT OF IRON CONTENT. WHAT SHOULD BE THAT IRON CONTENT? AND PLEASE TELL ME HOW TO MEASURE THE IRON CONTENT IN A PICKLING BATH CONTAINING HYDROCHLORIC ACID?
P. BHALAJEEbicycle mfgr. - Chennai, India
2002
|
A. Hi, The upper limit is about 80 g/l Fe assuming 15% HCl content. 1. Using commercial HCl a 50:50 solution in water will have a titration of 50 pts (1 ml sample, 0.1N sodium hydroxide, methyl orange ⇦ this on eBay or Amazon [affil links] indicator) 2. Ferrous iron content: 1 ml sample, add 10-20 mls clean water, few mls of 40% sulfuric acid, titrate with 0.1 N potassium permanganate ⇦ this on eBay or Amazon [affil links] to a pink coloration. Titre X 5.6 give g/l Fe. 3. Add pickling inhibitor to the acid to prolong bath life (Stannine LTP is suitable) R Roger Bridger- Croydon, U.K. 2002 A. 5 ounces per gallon maximum iron concentration. Total Fe analysis: sample 5 ml of bath, add 100 ml H2O, 5 gr Cadmium flakes, boil 5 min, cool and decant liquid in flask, wash Cd residue with H2O and add to flask, add 25 ml 20% ZnSO4 and 50 ml 10% H2SO4, titrate with 0.1 Normal KMnO4 to colorless pink. Total Fe = ml titrant x 1.489 x N. Guillermo MarrufoMonterrey, NL, Mexico 2002 |
A. All nonsense: Sorry.
You can go to 160 even 180 g/l Fe2+ in your Hydrochloric acid when you go down in your concentration of Hydrochloric acid.
I made a program for it. High HCl low Fe2+, Low HCl high Fe2+
Determination of Iron is made with 0.1 N Potassium permanganate: the use ml x 5,6 gives you g/l Fe2+ as Mr. Roger Bridger says. It's no secret.
process engineer - Netherlands
Ed. note: Thanks, John! But please use terms like "incorrect" or "inaccurate" because "nonsense" is a pejorative term, and we all work very hard here to avoid being insulting or unwelcoming :-)
Q. I have question to ask Roger Bridger who responded to P.Bhalajee. How did you come up with value X5.6?
Rosalina da Silva- Burlington, Ontario, Canada
2003
Q. Hi. I wanted to ask the same thing but I use iron-save so what should be the amount of iron g/l? I use ortho phosphoric acid with HCl from the tank & flux? Is it ok? or what?
Doaa Arafa- Giza, Cairo, Egypt
October 18, 2008
A. To
Rosalina Da Silva
Here is solution of *5.6
Actually it is multiplication of atomic weight of Fe and normality of KMnO4
55.845 * 0.1 = 5.58 to 5.6
- NAGPUR INDIA
February 2, 2017
Q. Maximum iron content in pickling HCl(50%)? We are one of the leading electroplating industries in Chennai (India). We want to know what is the maximum iron content allowable to operate and discard?
What is the simple procedures to check the iron content (ppm) in HCl (for pickling plant)
managing partner - Chennai, Tamil Nadu, India.
December 30, 2008
August 8, 2012
Q. I want to measure the accurate HCl concentration of the Pickling Acid which is used for steel pickling. I did titration of the pickling acid sample with sodium carbonate
⇦ this on
eBay or
Amazon]
by using methyl orange
⇦ this on
eBay or
Amazon [affil links]
indicator, it turns red color to orange color as end point but the problem is that the color cannot be sharply observed as to distinguish red-orange end point is difficult.
Usually when I do titration of 0.5 M HCl solution with 0.1 N sodium carbonate solution by using methyl orange
⇦ this on
eBay or
Amazon [affil links]
indicator at the end point the color disappears so I can easily distinguish the end point in this case, kindly tell me which indicator I should use so I can get a clearly visible end point to determine the HCl concentration in the Pickling Acid for Steel.
Or what is the most accurate or standard procedure to find out the HCl concentration and Iron content present in the HCl Pickling Bath of Steel.
What are the laboratory procedures/methods to measure Hydrochloric Acid (HCl) concentration and Iron (Fe) content present in the Pickling Acid samples of Steel Industry?
- Karachi, Pakistan
Q. For checking of iron content in pickling bath acid we use a buffer solution (0.5 g diphenylalanine + 120 ml orthophosphoric acid + 40 ml H2SO4, and dilute it into dm water. Then volume make up to 2.5 liter, but I have a question: What is the significance of 120 ml orthophosphoric acid? If we add only 100 ml or 75 ml what will be change?
SUBHADEEP GHOSH- JAMSHEDPUR, India
August 20, 2012
A. Iron content can be easily estimated from pickling or acid bath. The sample is to be mixed with distilled water and small amount of tin chloride and titrated against potassium dichromate of known strength.
Spectralab Instruments Pvt. Ltd. - Thane, India
December 1, 2012
Q. In acid Pickling solution I have Iron content 110 ppm and Chloride content 5910 ppm - it is correct?
Yovan Raman- Coimbatore and India
April 9, 2018
A. Hello Yovan!
5910 ppm is 5,91% chloride content, so it's around 6,1% in acid concentration. I think it's too low to work properly for steel. Iron around 110 ppm is very low (we use it until it reaches 100 g/l, around 100000 ppm), so if you want to use this pickling bath for steel, I would make the acid stronger.
If you don't want to use it on steel, please tell us more about your process and we may be able to help you.
Best regards,
TEL - N FERRARIS - Cañuelas, Buenos Aires, Argentina
April 9, 2018
Q, A, or Comment on THIS thread -or- Start a NEW Thread