
-----
S/S to Steel Electrolysis and Corrosion
My boss is concerned about welding of a S/S cleat to 1/2" Steel plate, welding to a Steel Bulkhead, then painted. What are the chances of Electrolysis & Corrosion?
USCGC Decisive - Pascagoula, Mississippi
A battery is built by placing two different metals in a conductive solution called an electrolyte. As soon as you connect wires (a conductive path) from the battery to a load (such as a lightbulb), the more active of the metals begins to corrode (oxidize or go into solution), releasing electrons to flow and power the lightbulb. In a standard carbon-zinc battery, for example, the Zinc metal
(Zn0) begins to coorrode into Zinc ions (Zn++), releasing electrons that flow through the wire towards the carbon rod.
Any time you have two dissimilar metals, a solid conduction path provided by the metals touching, and an ionic path provided by moisture, you will have some galvanic corrosion because you have created such a battery, as illustrated:
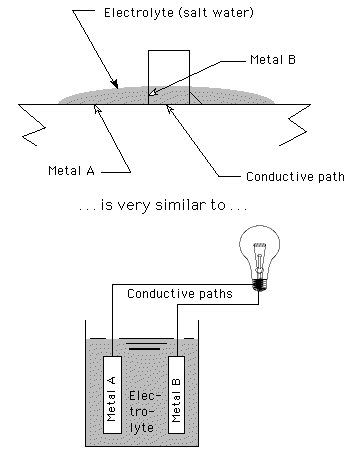
While dissimilar metals are often connected in a dry office or home environment, exposure to moisture, especially salt water, is a different matter. The welds and/or the steel around the welds are very likely to corrode excessively because paint really isn't impermeable.

Ted Mooney, P.E.
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
Q, A, or Comment on THIS thread -or- Start a NEW Thread