
-----
How-tos of Plating Compression Springs
Q. I need advice on plating some compression springs that are needed on a appliance that will see some humidity. I believe I should use galvanized material but some one suggested I use music wire with a phosphate and oil as it would be cheaper. I am afraid the phosphate would have a hard time getting full coverage and once the oil dried it would eventually rust...can someone advise how the galvanized material would hold up in this type of environment.
Thanks,
Bruce Swenson- Prospect, Kentucky
2001
A. The major problem with "coating" springs or other objects made of High Tensile Steel is Hydrogen Embrittlement . The most frequently used process that has been shown to produce little if any hydrogen embrittlement ( if used correctly ) is Mechanical Plating / Galvanising . I suggest that you look into having this process done I know there are several companies in the Detroit area that offer it as a service.
Regards,

John Tenison - Woods
- Victoria Australia
A. Bruce:
A thickness of 0.001" of zinc will give you from about 4 years of life in heavy industrial atmospheres, to 11 years if the environment is rural. If the location will be less than 80 ft from shore, cadmium is better though more expensive. Don't forget hydrogen stress relief after any electroplating is a MUST for springs.
Good luck,
Guillermo MarrufoMonterrey, NL, Mexico
2001
A. I think there is too much paranoia about Hydrogen Embrittlement during plating. It's necessary for the industry stalwarts to examine the way we have been doing things and getting out of this situation that we're too used to the problems we already have and cannot see the advantage of doing things in a better way.
I would not advise Cadmium, nor de-embrittlement for springs as the temper and strength of the springs may be compromised.
Rather I would advise mechanical cleaning to a fully bright condition by vibro-finish or sandblasting, then a light acidic dip (I use rinsewater from my Acid Zinc tanks - no acid) followed by Chloride zinc plating. This process causes no embrittlement and is safe for springs and I'd swear by it.
Regards,

Khozem Vahaanwala
Saify Ind
Bengaluru, Karnataka, India

2001
A. To Bruce Swenson - Prospect, KY --
The cost of the compression spring, the cost of the appliance and the budgeted amount that corrosion protection would need are factors that will determine what you will end up paying for the process you choose to apply.
Obviously you don't want the spring to outlive the appliance. So talk to your Friendly neighborhood plater for an answer.
10 microns acid zinc with a yellow chromate after a short 90 minutes De-embrittlement at 220 &C is a possible answer that can give up to 96 hours white rust in a 5 % salt spray test.

Asif Nurie [deceased]
- New Delhi, India
With deep regret we sadly advise that Asif passed away on Jan 24, 2016
2000
Help identifying what this spring was finished with
Q. Hi,
I'd like to have my throttle springs on my car refinished to look like new from the factory. I have a new throttle body with the spring from the manufacture. The used springs on my car have turned a red/brown color, some areas of the spring are even showing shiny metal where the finishing material has worn off.
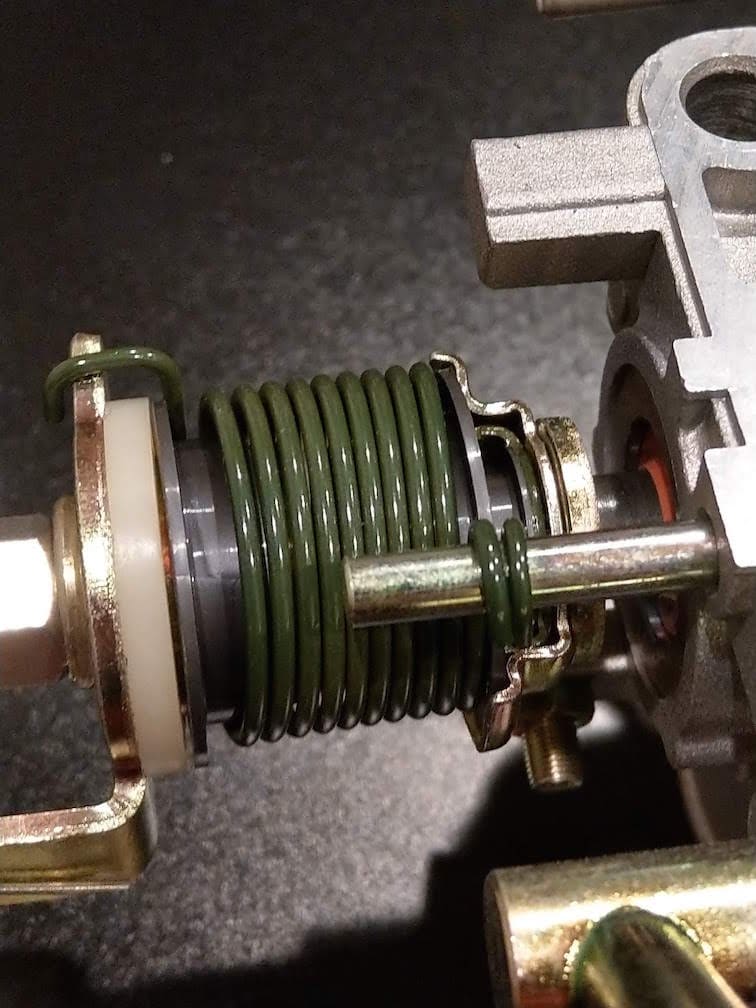
Can anyone help me try to determine what material/process may have been used to finish the spring? I appreciate all the help anyone can provide.
Greg Lussier- Sammamish, Washington USA
November 29, 2018
A. Hi Greg. If you identify the year, make, and model of your car someone may be able to help. Trying to judge what material something is from looking at it, let alone a photograph, isn't the way forward :-(
It rather looks like cadmium plating with a better than perfect olive-drab chromate conversion coating, but my guess is that it's actually an electrophoretic lacquer (CED coating) perhaps on a stainless steel or zinc plated spring.
P.S.: I had to reduce the size of your photo and use "lossy compression" for presentation on this site, but if you're not a professional photographer you ought to be; the pic was stunningly perfect :-)
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
November 2018
November 29, 2018
Q. Ted, Thank you for the response.
I do enjoy taking photos but luck may have been on my side with that one as it was taken with a cell camera. haha
The car is a 1996 Nissan. I've noticed a lot of their models from the same era (90-2000) have the same coating/color on their throttle body springs.
I'll provide photos of the used units as well to show the brown color it turns once age has taken its toll:


- Sammamish, Washington USA
A. Hi again Greg. My father in law was an outstanding amateur photographer too. People sometimes asked what kind of camera he used. He said it was from the same company that made Shakespeare's pens :-)
We have other threads here (topic 58797, topic 48993) where people have inquired about a "brown chromate". Nissan would not have used cadmium plating in 1996, so I have to revise my guess to zinc plating with an olive green chromate which eventually turns brown and wears off.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted is available for instant help
or longer-term assistance.
December 2018
Q, A, or Comment on THIS thread -or- Start a NEW Thread
