
-----
Copper electroforming problems
< Prev. page
(You're on the last page)
for Artisans, Jewelry, Crafts

avail from eBay, AbeBooks or Amazon

avail from Amazon

avail from Amazon

avail from eBay, AbeBooks or Amazon

avail from eBay, AbeBooks or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
Q. Hello. I am having a problem with getting any substantial amp. readings or plating occurring. I have a 7 gallon tank with all Technics equipment. I'm running copper foil test pieces in the tank. 3 @ 3"x 3". I have the volts up to 7.5 and it is only pushing .5 amps. The pieces are salmon colored. I hadn't used the set up for over 6 months, much of the water evaporated so I replenished it with distilled water. Copper anodes were out of the tank during this time.
Any ideas? Thanks
- High Falls, New York
September 20, 2018
A. Hi Jamie. Put the leads of a V-O-M directly on the anode and your test pieces to insure that the problem is neither with the rectifier nor dirty connections. If the pieces are receiving anywhere near 7.5 Volts that should be way more than enough, and probably quite excessive -- which means that the problem is the anodes or the solution.
I don't know what you have in the way of chemical analysis or test equipment, but if you do not have a Hull Cell ⇦ huh? and you aren't able to do any analysis, and cleaning the anodes doesn't help, you might be limited to replacing the solution. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi, I am trying to electroform with copper but when I pull it out of the bath and rinse it off, after drying it seems to develop a bright rainbow iridescent finish. any idea why this may be?
When electroforming with copper, are there certain shapes that are easier to "burn"? I have been trying to electroform a pinecone. I wrapped a wire in a coil around the pinecone and it seems to keep turning matte brown. other things I've had in the bath at the same time however have turned out fine so I'm assuming it has something to do with the shape/ surface area or my wrapping technique?
- columbus, ohio, united states of america
October 1, 2018
Q. Hello! I'm quite new to electroforming (about a month) and I'm grateful to have come across this forum :) I've managed to work through a lot of my problems through research and trial and error but the latest problem is really stumping me.

I set things up and mixed my bath according to Jason Welsh's methods. I have his book and I've watched a ton of his YouTube videos. The bath is made with distilled water, root killer as the copper sulphate, and battery acid
⇦ on
eBay
or
Amazon [affil link]
(not using a brightener). It's about a half gallon of solution, and so far I've been making small pieces, surface areas in the neighborhood of an inch or so.
The problem I'm having is that instead of getting a nice thick coating over time, my pieces will get a very thin coating and then develop these super sharp copper 'spines' all over. Not a very friendly texture for jewelry!
Maybe I'm not using the right terms in my Googling but I'm having trouble finding any information on this phenomenon. I did read something to suggest that sharp textures could be the result of copper-oversaturation, so I tried diluting with distilled water & sulfuric acid, but the next piece still came out spiky. I'm at a loss for what to try next!
Any clues would be very much appreciated!
- Denver, Colorado, USA
December 6, 2018
A. Hi Emily. Jason has great instructor skills, speaking slowly & clearly, and repeating things.
But some important points:
-The right way to electroform is with properly formulated addition agents including brighteners. Although brightener is expensive, almost all platers and electroforming shops uses it to prevent the "treeing" you illustrate.
- Electroforming faster (higher current), although it will not prevent treeing, may at least encourage "lumpy" trees rather than "spiky" trees because they'll "burn" away before they get quite so thin.
- Jason has a youtube video titled "Electropolishing Copper, the End of Brightner (sic) in electroforming" where he acknowledges these trees and removes them.
- A really important point, though, is that Jason is good at liking the look he gets :-)
Wavy, lumpy, uneven electroforming may be fine for the stuff he's doing, lending an artistic one-of-a-kind appearance; and it may be a fine look for your items too -- it's a matter of what you're looking for. But electroforming without brightener, and then trying to fix it by electropolishing or tumbling the spikes back to lumps isn't for precision items. Try to even imagine a stamper for vinyl records or CDs made by such methods.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi, I am fairly new to electro-forming and before I ask my question I want to make any potential answering members aware that I have no interest in mysterious pre-made chemical solutions as far a solution for my problem is considered.
First off I am working with a home made solution derived from Jason Welshes free Youtube tutorial (copper sulphate) and my electrodes are high purity copper. Now my Problem is that I have not been able to achieve a shine on any of the pieces I've electroformed. They have all come out of the bath with a flat salmon color and have poor adhesion. I Have tried different levels of current, this only changes the texture of the deposits. I have also added a simple home made brightener (polythylene glycol) into the solution after I carbon filtered. I don't have a pH meter so I cannot determine if the solution is of the correct pH but I do know that I have not added much in the way of water to it and I'm sure it has experienced evaporation. I plan on adding some distilled water but if that doesn't help what are my options?
- hamilton, Ohio, united states
January 5, 2019
![]() Hi Ethan. Everyone is very welcome to choose their own interests; I wouldn't dare challenge that, and no problem at all that you want solutions that don't involve proprietaries!
Hi Ethan. Everyone is very welcome to choose their own interests; I wouldn't dare challenge that, and no problem at all that you want solutions that don't involve proprietaries!
... but since I've already repeatedly said that I feel they are the only way to go, and you are clearly stated that you have no interest in my way, peace out :-)
Hopefully another reader will address your questions in the way that you wish.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. We are setting up a 20 amp rectifier to a 5 liter tank and it has been a nightmare to find instruction on how to set Amps and Volts. We often find contradictory advice. There is a fine and coarse dial, but what do we do first, turn on Amps and adjust Volts or the other way around? I've read 0.1 amp per square inch and 1 to 2 Volts but not sure in what order to do this. We have tried electroforming with turning Amps up until Volt reading was 0.5 and and Amps read 0.2 an hour later turned Volts to 0.6 and Amps went up to 0.3 but not much plating occurs on 5 square inches of surface.
Breeta Toma- San Jose, California
March 12, 2019
A. Hi Breeta. Although you'll never get away from finding contradictory advice, understanding the principles is a good first step; then you can decide what seems reasonable and try it :-)
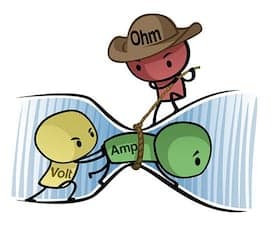
(courtesy of www.build-electronic-circuits.com)
The thing is, amperage and voltage are not independent; they are completely locked together by Ohm's Law:
Amps = Volts / Resistance
Any given setup will have a given electrical resistance based on things like the pH, concentration & temperature of the solution and the distance between anode and cathode. Then if you increase the voltage, the amperage will increase; if you decrease the voltage, the amperage will decrease proportionately. Yes, some power supplies let you control amperage -- but when they do that, they are simply letting you choose to control the amperage, by having the power supply put out however much voltage it needs to in order to deliver that much amperage.
It sounds like your particular power supply has a switch allowing you to control by voltage or by amperage, and then a pair of coarse & fine adjustment knobs which do the double duty of controlling either the voltage or the amperage depending upon whichever you selected with the switch.
If the area of your part is 5 square inches (remember to include both sides), and you want to try plating at 0.1 Amps/square inch, set the switch to Amps, adjust the knobs until you read 0.5 A (which is quite low for a 20 Amp rectifier) and the voltage will adjust itself whatever it needs to, probably to a volt or two.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
![]() Thank you so much, and the graphic is especially helpful. Had my first success and think we'll be off and running. We set the Volts at 1.5 and that did the trick.
Thank you so much, and the graphic is especially helpful. Had my first success and think we'll be off and running. We set the Volts at 1.5 and that did the trick.
- San Jose, California
Adhesion problem with Copper electroplating/electroforming
Q. I'm hoping someone with more experience than I may be able to shed some light. I am very new to electroplating and enjoying this experimental stage however I'm not getting great results thus far.
I am endeavouring to plate copper rings with gemstones. I have used epoxy putty to mould the stone to the ring base and once dry coated that with a conductive paint. (It's worth noting I have tried both a home made graphite paint and a commercial one with the same result)
For the power source I have two 1.5v batteries with alligator clips - negative to cathode positive to anode.
The bath is copper sulphate.
I have a copper coil anode inside the bath.
The piece to be coated is suspended via a copper wire wrapped on a dowel (wood).
My problem is the plating - after several hours the tiny copper particles are attracted to the cathode but just form a muddy layer that doesn't really stick.
I must mention I have tried several different power sources, bath solution strengths and anode pieces. I'm getting the same results each time.
Any wisdom is greatly appreciated. I do apologize for the long post but wanted to provide all the information.
- Batemans Bay, NSW, Australia
March 30, 2019
A. Hi Dannelle. A hobbyist/experimenter like yourself is unlikely to have any substantial analytical tools with which to troubleshoot ... yet you need to start figuring out whether the problem is the plating solution and your operation of it, or something to do with the ring and putty and metalizing process.
So I think the way to start is to get a nice piece of brass (maybe an old key?), clean it very thoroughly while wearing gloves, rinse it, acid dip it for activation, rinse it, and attempt to plate it. Once you can plate the key fine you can move on to trying your ring again. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Have just filtered my electrolyte to try and fix this problem, still the same issue.
My parts are a wax mold painted with conductive copper paint.
Solution is 3 gal distilled water, 2.5 pounds copper sulphate
⇦ on
eBay or
Amazon [affil link] 1 pint sulfuric acid, using regulated power supply in constant current mode -- same results when 1 amp or 2.5 amps.

The parts copper deposit is very grainy or nodular and kinda crumbly.
What am I doing wrong?
VINTAGE MUSEUM - Machesney Park, ILLINOIS, USA
April 10, 2019
Q. Try 200 gms copper sulphate/ 30 ml sulfuric acid / 1 lit distilled water solution. Use copper wires only.
Cathode anode distance 10 cm at least. Keep current density as low as it is possible. Hope it helps and good luck!
- Zagreb , Croatia
Q. PLEASE DOUBLE CHECK TO SEE IF I'M CORRECT FROM WHAT YOU GAVE ME.
3 gallons of water would require 5 lbs of copper sulphate and 1.43 cups of sulfuric acid.
I also see some forums saying to use brightener... What is that?
VINTAGE MUSEUM - Machesney Park, Illinois
A. Hi Chuck. Yes, your 5 lbs and 1.43 cups for 3 gallons sound right, but I think you'll need to get comfortable with "grams per liter" because asking people from Europe to convert their answers to pounds per gallon and cups per gallon for you won't work well; those are terms that many Europeans have probably never even heard in their life :-)
Goran's numbers would be for 96% or 100% sulfuric acid, not battery acid -- what you are using?
"Brightener" means organic addition agents which shield the high current density areas from plating in order to smooth, level, and brighten the plating. It's usually not a generic chemical but a system of proprietary ones from a vendor of acid copper plating solutions. Some people, like Ethan Rowlette above, and Jason Welch in his youtube videos, are confident that such addition agents are unnecessary; others, including me, think you should buy a proprietary copper plating solution from a vendor of proven solutions rather than trying to formulate your own plating solutions.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Yes I am using battery acid as it is what was listed in the recipe I had found on line.
The density of both the copper sulphate and the acid were about half in my recipe compared to these suggestions.
Will be looking for a source for the brightener.
Then the question will become how much of that to add.
Chuck
VINTAGE MUSEUM - Machesney Park, Illinois
A. Chuck Jansen:
That's a really ugly copper deposit. It's going to take more than some brightener to make it useful.
I suspect you have used some poor quality chemicals.
If you really want to pursue this, contact a supplier of plating chemicals; a few of them deal in hobby-sized quantities.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
Q: Hi.
Firstly, sorry for my english. I want to prepare about 300 [µm] thickness surface of copper on nickel plate by electroplating. My bath is made from:
-200g of CuSO4 * 5H20
-50g of H2SO4
It is working at 298 °K [25 °C, 77 °F]. The voltage between Pt anode and nickel cathode is 1.7 [V] and current is 0.01 [A]. The nickel plate is about 0.0416 [dm2] = 0.645 [in2]. After 24h of plating I have rough copper surface What should I change?

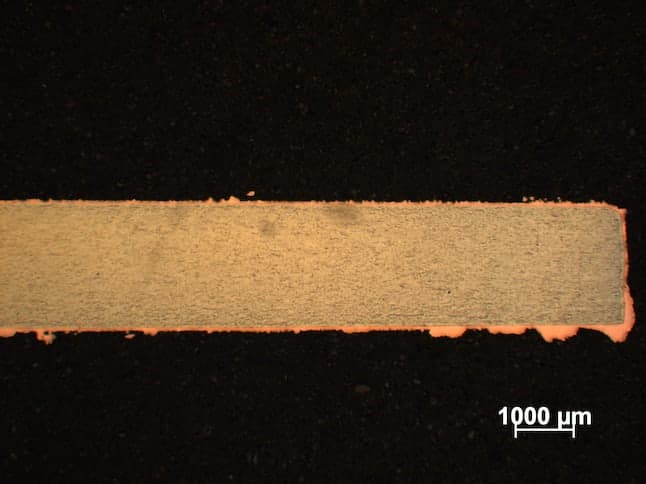
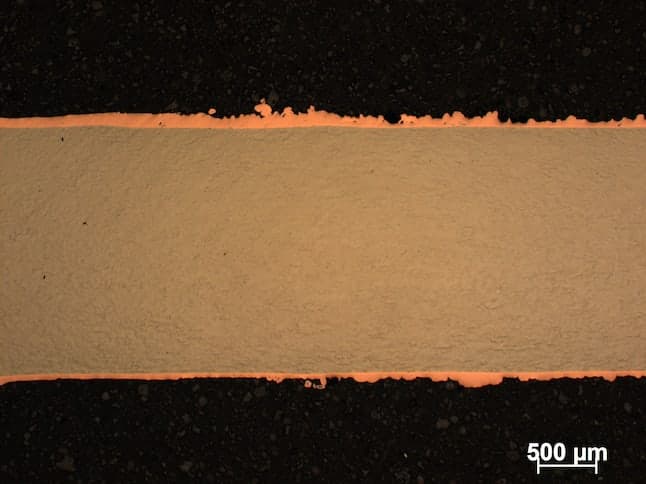

- Cracow, Lesserpoland/Poland
June 11, 2019
A. Hi Szymon. Phosphorized copper anodes might be less trouble than platinum, but I believe platinum will work. Roughness can be due to a few different things, but you should filter the solution and have 50-100 ppm of chloride. As Jeffrey mentions on an earlier posting, low quality chemicals can be the problem too. Is the beaker [beakers on eBay or Amazon [affil link] sitting on a magnetic stirrer? If so, are you stirring the solution during the plating time?
Commercially available plating processes will outperform home brew. You haven't mentioned activation of the nickel yet.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Bright acid copper over electroformed copper?
Q. Pretty quick question... I've been pretty good results with my electroforming, but it is a little dull. Can I use a bright acid copper bath to build up the thickness and then hit it with a polishing wheel? Or should I be working the amps and timing to get a better finish from the bath? Thanks
Ray Walker- Salt Lake City, Utah
August 16, 2019
A. Yes, you can use a bright copper bath, but the deposit will be less ductile. This may or may not be an issue, depending on end use.

Jeffrey Holmes, CEF
Spartanburg, South Carolina
![]() Thanks for the feedback Jeffrey, the piece is just meant to be decorative so it being less ductile shouldn't be a problem.
Thanks for the feedback Jeffrey, the piece is just meant to be decorative so it being less ductile shouldn't be a problem.
O.C. Tanner - Salt Lake City, Utah, USA
Q. I am having to scrub my wire coil anode that's in the bath every hour which is super annoying. Any advise on how to avoid this? The wire gets dull and then it stops forming and my rectifier shows no amps.
Carly VezzaniEarth and stone wrapz - Milwaukie, oregon, usa
March 30, 2020
A. Hi Carly. Just to make sure this doesn't go off the rails over a typo, you are using a coil of copper wire as your anode, and your anode starts getting dull and covered with something that stops it from dissolving and stops the current flow? The object that you are electroforming still looks good?
Presumably you are using copper sulphate and sulfuric acid as your electroforming solution? Any idea on the acid content or pH or anything? What are you electroforming?
If you search the site for "passive anodes acid copper plating" you will see a number of discussions about the phenomenon that you are encountering. Good luck.
Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I'm just starting to electroplate/form and I'm using organic pieces. I mixed up my own batch of copper solution as per Jason Welsh's recipe. Pieces are coming out shiny sometimes, but mostly dull. I'm going by 10 amps per sq inch of surface. I added one drop of brightener from Rio. I do filter the solution before reusing it.
Is there something I'm missing? Organic pieces are sealed in an acrylic lacquer spray then dipped twice to seal. Should I check the pH of the solution and if so what should it be at?
Thanks and sorry for the long winded question.
- Lomita California
July 7, 2020
A. Hi Mitch. You're certainly not plating at 10 Amps per square inch or even 1 Amp per square inch. Let's start by getting the right number for that :-)
Maybe 10 Amps per square foot?
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Sorry, I meant to say .10 amps per sq inch.
- Lomita California
A. Hi again. That current density sounds about right, maybe a little high for electroforming, maybe a little low for bright copper plating.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Copper is growing over ring's stone during electroforming
Q. Hello!
I just got a new power supply from Amazon, and I'm really happy with it. It seems to be doing a much better job than my last one, perhaps too much so! I am now having trouble with copper forming over a stone (angel aura Quartz) in places I have not painted with conductive paint. I have my power supply set to 0.1 Amps (it is a small ring) and I'm using the Midas bright copper electroforming solution. The solution is fairly new, has only been used once before. I pulled the ring out when I realized it was forming copper over the stone and pulled off the thin layer that had formed. I thought perhaps the metal in the angel aura coating was perhaps the culprit although I've never had that happen before! I coated the stone in decoupage and put it back in and same thing, the copper formed over the decoupage. I pulled it out again and tried a coat of nail polish and placed the ring in the bath but same thing, copper is forming over the nail polish. Do you know what might be happening?
Thank you so much!
Alex
- Dana Point, California
August 8, 2020
This meeting place welcomes Q&As, photos, history, & interesting tidbits.
Please engage with other posters
• When people show interest in each other's situations, the page quickly becomes a fun & informative learning experience for everyone !
• When people show no interest in other people's postings, and just post their own, it often quickly deteriorates into a string of unanswered questions 🙂
Q. Hello, I am totally new to electroforming and having a terrible time because none of my pieces come out correctly. I am afraid that there's something wrong with the preparation. Could you please confirm that I need to apply a conductive paint (I use liquitex because water based) and when dry a coat or two of copper pigment powder mixed with few drops of conductive paint and a bit of distilled water?
Thank you!
AF
- New York New York
October 28, 2020
Q. I have a ring I electroformed I followed the instructions and painted only where I wanted my copper to go and sealed my stones with latex. But the copper totally covered my stones, is there a way to remove it now so I can have my stones show?
Patsy Solomon- Coeur d'Alene, Idaho
February 19, 2021
Q. I am extremely new to electro forming I have my red wire connected to the copper anode and my black wire attached to the copper wire that is holding my piece (pendant covered in graphite paint) I have watched several videos on how to set up my rectifier however my vault do not seem to go up no matter what dial I move. The amps will go up but the voltage does not. What am I doing wrong? Also is it normal for your piece to be covered in blue stuff from the bath?
Madison Warren- Lebanon Ohio
March 19, 2021
We'd like to give credit for this graphic explaining Ohm's Law (A = V / Ω) but see it on many websites without info of whose work it actually was :-(

A. Hi Madison. The voltmeter on your rectifier is apparently defective because the amps is not increasing unless the voltage also increasing. If you have a portable multimeter you can check the voltage between the red and black wires and verify this.
If your piece is covered in blue stuff perhaps your graphite paint is rough and spongey.
I realize that people would like to swallow the whole elephant in a single bite, but I think everything will go much faster for you if you do some trial plating on metal first. Plating/electroforming is hard enough to learn without adding the complication of a graphite paint attempt at metallization.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hi.
I don't know what's wrong with my electroforming tank. I am now getting these random streaking/ erratic ridges on the surface of my samples.
I made some silly decisions and messed with it. it was over-saturated so I added 1L distilled water to 9L tank, at the same time I filtered the solution through a house filter. Did not come out completely clear - still a bit murky. Additionally, I made another 2L of the solution up and added it because I needed a bigger bath (however it's important to note I did not add any chloride because I am trying to achieve texture).
My pH is now around 0.8. I'm getting about 2.0 amp to 1.0 V.
Ran a test piece overnight and got a lot of texture. Ran 20 min test piece this morning, streaking still evident, and pieces are incredibly brittle.
Also worth noting I have no brightener in the bath. So at least that removes one of my troubles.
- Eindhoven Netherlands
April 9, 2021
Q. Hello! First of all I'm from Spain and hard to find a lot of the items and materials needed.
I just want to start electroforming, bought an Alkaline copper plating solution 1L, don't know if I should get the acidic one.
I have my rectifier that goes to 30V- 10 Amp.(I use it to a very low V/A)
I have bare copper wire in a small plastic container, where the 1L of solution fits.
The thing is I ordered the conductive paint, as I want to make some jewelry with no conductive materials, bus as now I don't have it yet, so I tried the Graphite and Acrylic paint method but... it doesn't work, like at all. I already putted in some metal pieces to know if they would plate/electroform and for sure they do, also the copper wire I hang my pieces from gets dark or so on when fitting at first the rectifier.
So... am I not using the right homemade conductive paint measurements/recipe? I did a lot of tests with different ratios and nothing works. ALSO, IMPORTANT: I just let the paint dry and the put it in the bath, I'm not sure if I should do lot of layers and wait like hours or a day for the graphite paint to dry or if is just because I should just wait for the real deal, the good conductive paint?
Thank you before hand!
- Alicante Spain
April 16, 2021
Q. I am experiencing white anode. After I used some muriatic acid... Also the blue is losing color... Copper forms on objects a pale salmon and very smooth. Can my bath be saved? If so what do I need to do. The easiest way would be to start over but I want to learn how to fix mistakes.
Tonie Knutz- Curtis, Washington
April 18, 2021
Q. Hello my name's Eli and I just started electroforming and on some of my pieces the copper is not very thick and I can't figure out why. Any suggestions? As well, will my power source going off affect my plating? I came out to turn up my power and my rectifier was turned off!
- Everett Washington
June 9, 2021
A. Hi Eli. Since you just started electroforming, please see our FAQ on Faraday's Law which will answer your question on why your copper is not very thick.
It is hard to say without any qualification that the power being off is not a problem, but for copper and with reasonable off-times, it's probably not a problem. If it was nickel it would be a major problem.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. I am having the same problem as the original poster from 1995: I cannot get copper to adhere to conductive paint (purchased graphite paint in my case). I have successfully plated brass, nickel, and whatever metal is on shiny gold colored fishing line hook connectors.
I have even placed these materials in the bath at the same time and only the conductive paint didn't work. I can see it bubble and it forms some copper built up but when i went to finish it the copper came right off; even using the finest polishing compound by hand.
i also tested the conductivity of the paint in several places. it carried a full voltage and had a resistance of 8-11 kOhms.
Any suggestions on how to get copper to stay on conductive paint?
Thanks
inventor/artist - roseville, California
July 11, 2021
A. Hi John. You can try a much thinner layer of your paint; it sounds like it's shearing. Other that that, a different type of conductive paint seems indicated.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Newbie here. I'm trying to make a copper electroplating solution. I'm using white vinegar ⇦in bulk on eBay or Amazon [affil link] and salt. Power supply is 12v 2.5 amps. My issue is huge chunks of copper flakes are falling off into my solution. So is white oxidation.

Hobbyist newbie - Kentucky
November 24, 2021
by William Safranek

on eBay or Amazon
or AbeBooks
(affil link)
A. Hi Dan. Our FAQ "How Plating Works" explains copper plating with vinegar and salt ... but the thing is, that page is about introducing school children to electroplating using safe kitchen ingredients. It allows them to produce a very thin but obviously copper colored deposit onto things for the purpose of demonstrating the science. But it is not easily extended to actual robust, functional, copper plating or copper electroforming. It's a solution and operating conditions capable of transporting some ions from the anode to the cathode, but not a process solution and operating conditions capable of robust plating.
There are whole books like Safranek just on the effects on the deposit of altering the acid used, the concentration, the temperature, etc.
Also, your voltage and amperage are at least 5X what would be reasonable for good plating even with a more capable solution and better operating conditions. So what you are depositing with the terrible 'burning' you are seeing would be expected -- there's no way enough ions can be present in your operating scenario to even begin to accommodate the number of electrons that 12V & 2 Amps is delivering; please see our FAQ on "Faraday's Law".
Look up a copper sulphate plus sulfuric acid plating solution and reduce your voltage 5-10 fold and get back to us please.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
November 2021
for Artisans, Jewelry, Crafts

avail from eBay, AbeBooks or Amazon

avail from Amazon

avail from Amazon

avail from eBay, AbeBooks or Amazon

avail from eBay, AbeBooks or Amazon
(as an Amazon Associate & eBay Partner, we earn from qualifying purchases)
Q. Hello! I've been making jewelry via Copper Electroforming for awhile. I stopped for a few months due to needing new solution. I made the new solution via copper sulphate, battery acid ⇦ on eBay or Amazon [affil link] , and distilled water exactly like I've done in the past.
However now, my rectifier times out or just isn't conducting anything? I just have a scrap piece of copper suspended in the liquid as a test but I can't get my rectifier to work. I've tried two rectifiers and two solutions. Tried plugging them into a different outlet as well.
Any tips to troubleshoot this?
- Florence, SC
April 19, 2022
A. Hi S. I would have assumed there was something wrong with the rectifier, but something wrong with two rectifiers sounds unlikely. Make sure the input voltage switch on the back is correctly set, then test the rectifier's voltage controls and current controls per the instructions. If it performs correctly, maybe the rectifier is set for 0 Amps constant current; make sure it's set right.
If you are sure the rectifier is okay and you are operating it right, then: I am not confident that this is the problem you are having, and your solution doesn't look super saturated, but as food for thought, a copper plating solution which is too strong will not conduct. Some people call it the "anode going passive".
I am not a theoretical electrochemist able to fully explain it, but it seems that a very saturated solution causes a film to build near the anode which can't carry electricity.
So, after you are confident the problem isn't the rectifier, I'd suggest taking the anode out and scrubbing it with 00 steel wool ⇦ on eBay or Amazon [affil link] , and while it's out try diluting the solution 50:50 with DI water before putting the anode back in. Let us know if it changes anything.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Q. Hello! I am having trouble with my 9 quart igloo homemade tank that I use for copper plating 3D models I make. My issue is I recently had to dump the old solution out due to it having sat inactive for about 6 months ... so I made a new solution using water, copper sulphate, a little battery acid
⇦ on
eBay
or
Amazon [affil link]
, and a little table salt. The solution is plating the copper wires I'm hanging pieces from in the tank, but not the pieces themselves. I homemade my paint 10 or so months ago with graphite powder, modge podge, and water. I painted the pieces months ago as well. I am confused because the old solution worked fine for months and plated my stuff beautifully, but now I can't understand what I'm doing wrong now...
Thank you for your time!
hobbyist - Indianapolis, Indiana
July 29, 2022
A. Hi. The way to start is probably to take a Hull Cell panel or other very platable substrate and try to plate that because it will tell you whether your plating setup is the problem or the substrate is the problem.
Luck & Regards,

Ted Mooney, P.E. RET
Striving to live Aloha
finishing.com - Pine Beach, New Jersey
Ted can be retained for immediate
answers or long term project help
Ed. note: Hi Gentle readers. Please remember that this is a mutual-help public forum, not a consulting service. Please try your best to answer a question not just post your own. Thanks!
Q, A, or Comment on THIS thread -or- Start a NEW Thread


